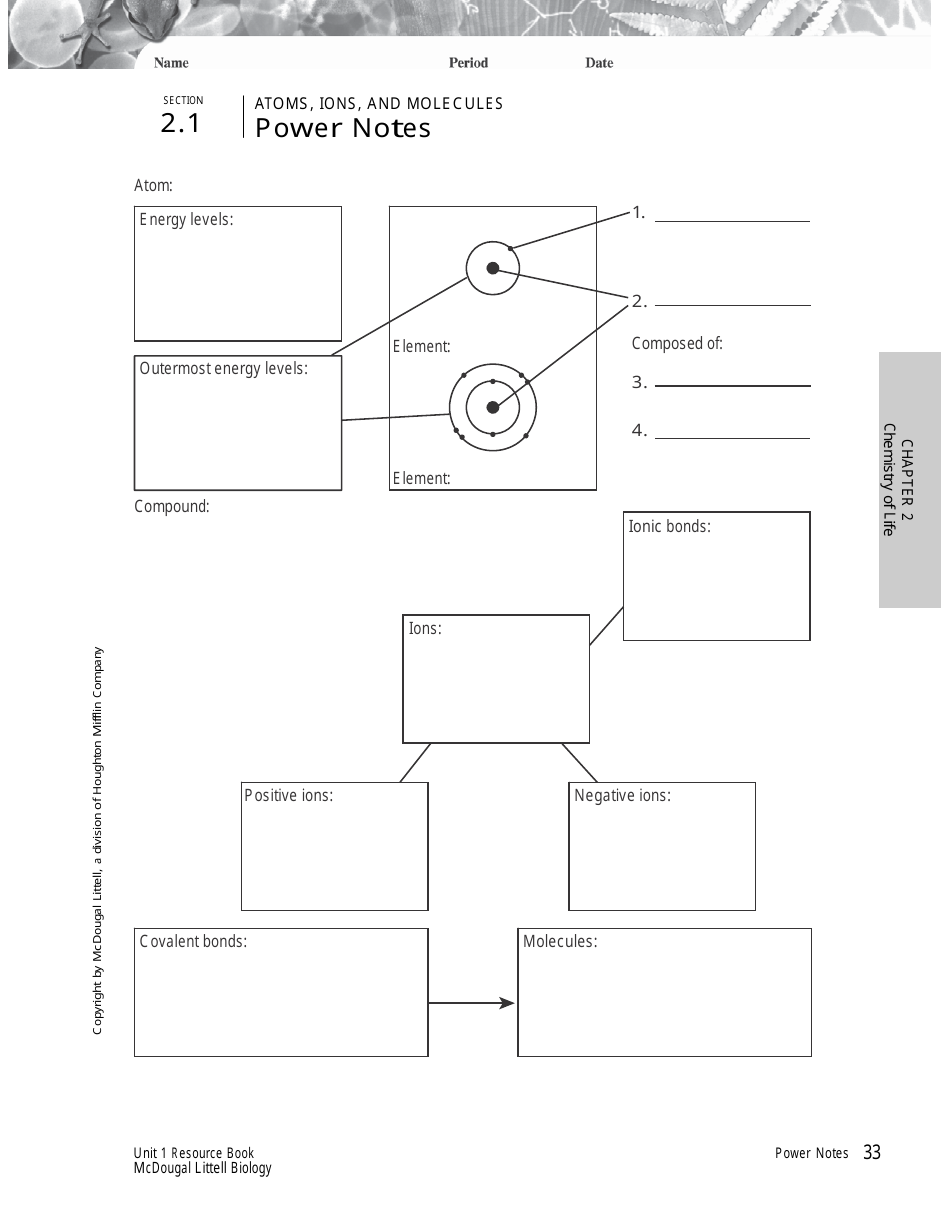
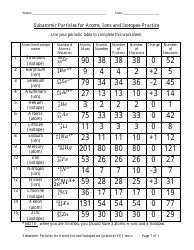
Atoms, Ions, and Molecules Study Guide - Section 2.1, Chemistry of Life Study Guide Book, Mcdougal Littell
The "Atoms, Ions, and Molecules Study Guide - Section 2.1" is a part of the "Chemistry of Life Study Guide Book" published by Mcdougal Littell. It is designed to help students understand the concepts of atoms, ions, and molecules in the context of chemistry.
FAQ
Q: What is an atom?
A: An atom is the smallest unit of matter that retains the properties of an element.
Q: What is an ion?
A: An ion is an atom or molecule that has gained or lost one or more electrons, leading to a positive or negative charge.
Q: What is a molecule?
A: A molecule is a group of atoms bonded together, representing the smallest fundamental unit of a chemical compound.
Q: What is the chemistry of life?
A: The chemistry of life refers to the chemical processes and reactions that occur within living organisms.
Q: Who is the author of the Chemistry of Life Study Guide book?
A: The author of the Chemistry of Life Study Guide book is McDougal Littell.