This version of the form is not currently in use and is provided for reference only. Download this version of
Form HLTH5368
for the current year.
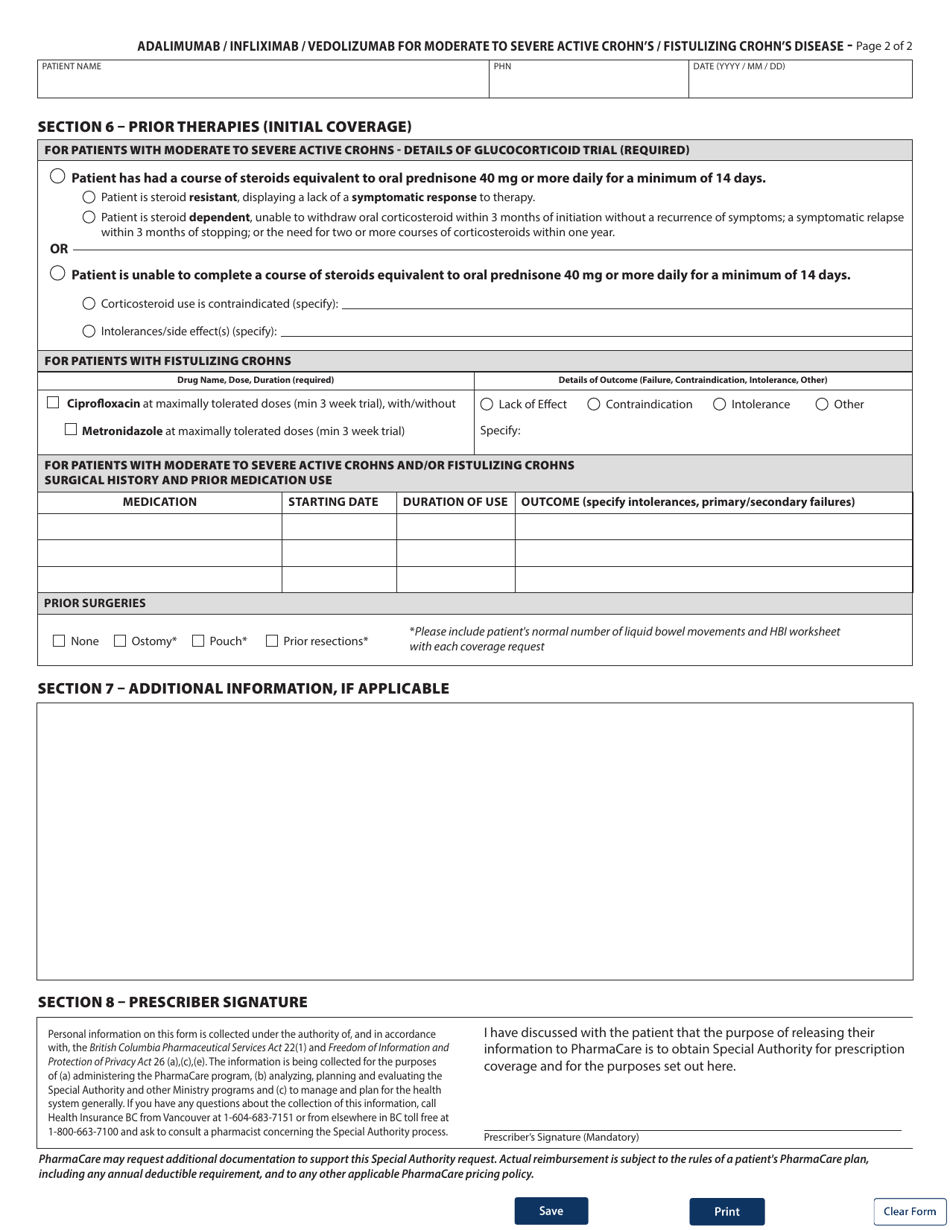
Form HLTH5368 Special Authority Request - Adalimumab / Infliximab / Vedolizumab for Moderate to Severe Active Crohn's / Fistulizing Crohn's Disease: Initial / Switch Coverage - British Columbia, Canada
Form HLTH5368 Special Authority Request is used in British Columbia, Canada for requesting coverage for the medications Adalimumab, Infliximab, and Vedolizumab for the treatment of Moderate to Severe Active Crohn's Disease or Fistulizing Crohn's Disease. It is used for both initial coverage requests and requests to switch from one medication to another.
The form HLTH5368 Special Authority Request for Adalimumab/Infliximab/Vedolizumab for Moderate to Severe Active Crohn's/Fistulizing Crohn's Disease in British Columbia, Canada is typically filed by the patient's healthcare provider or specialist.
FAQ
Q: What is the purpose of the form HLTH5368?
A: The purpose of the form HLTH5368 is to request special authority coverage for Adalimumab, Infliximab, or Vedolizumab for moderate to severe active Crohn's disease or fistulizing Crohn's disease in British Columbia, Canada.
Q: Who is eligible to use the form HLTH5368?
A: Patients in British Columbia with moderate to severe active Crohn's disease or fistulizing Crohn's disease are eligible to use the form HLTH5368 to request coverage for Adalimumab, Infliximab, or Vedolizumab.
Q: What medications are covered by the form HLTH5368?
A: The form HLTH5368 covers Adalimumab, Infliximab, and Vedolizumab for the treatment of moderate to severe active Crohn's disease or fistulizing Crohn's disease.
Q: How can I submit the form HLTH5368?
A: The form HLTH5368 can be submitted to the BC PharmaCare Special Authority office by fax or mail.
Q: What is the coverage criteria for the form HLTH5368?
A: The coverage criteria for the form HLTH5368 includes having a diagnosis of moderate to severe active Crohn's disease or fistulizing Crohn's disease, previous treatments failure or intolerance, and meeting specific clinical criteria.