This version of the form is not currently in use and is provided for reference only. Download this version of
the document
for the current year.
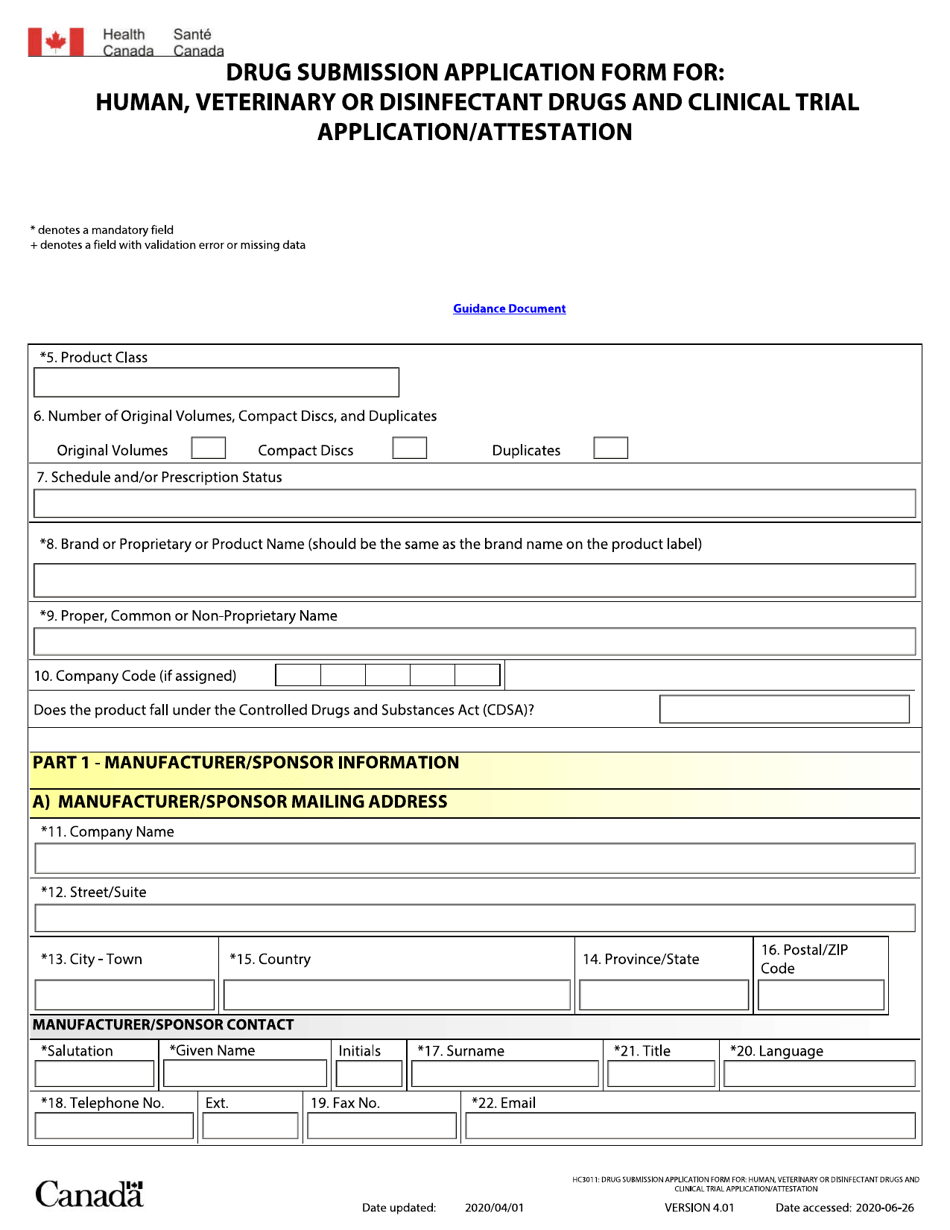
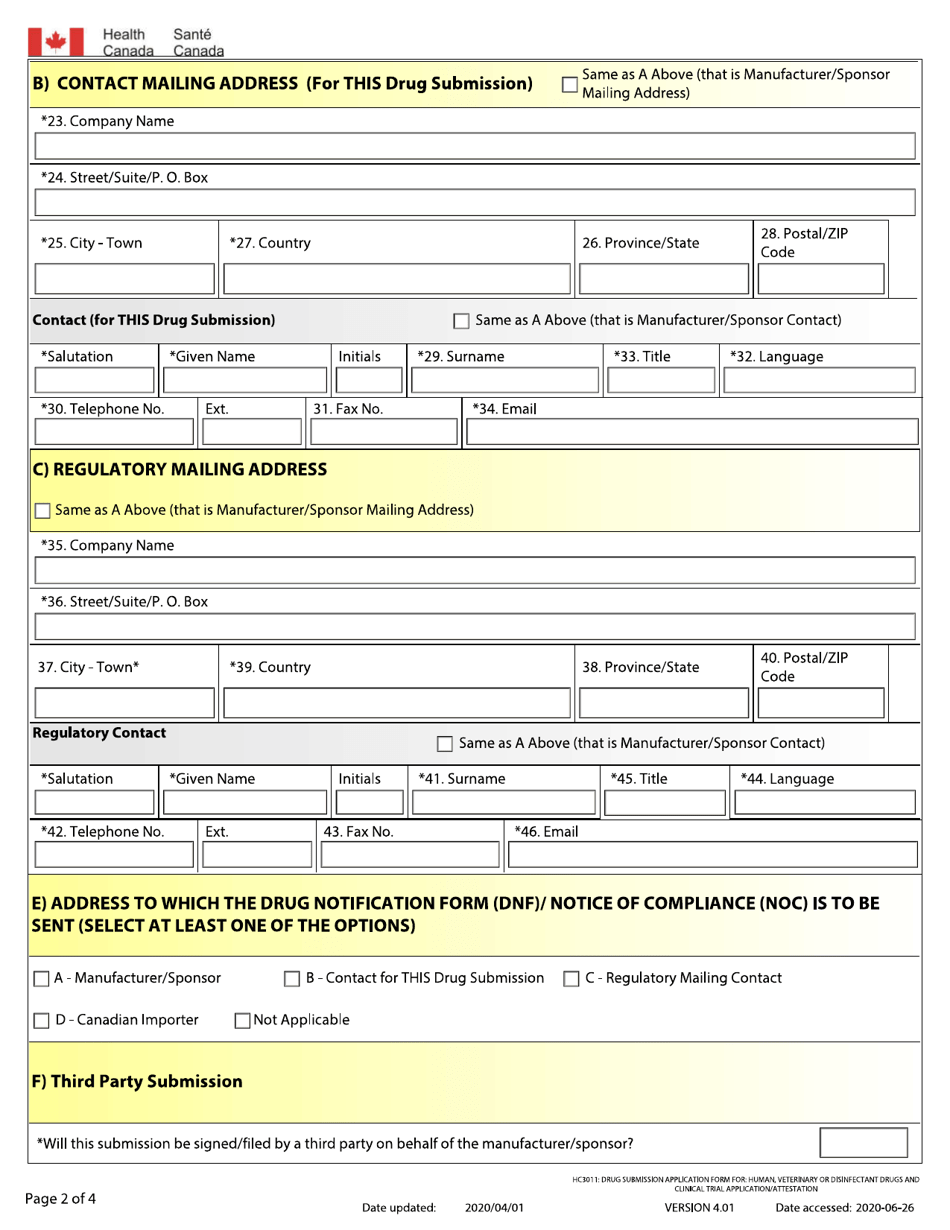
Drug Submission Application Form for: Human, Veterinary or Disinfectant Drugs and Clinical Trial Application / Attestation - Canada
The Drug Submission Application form in Canada is used for both human and veterinary drugs, as well as disinfectant drugs. It is also used for submitting clinical trial applications and attestations.
In Canada, the responsibility for filing the Drug Submission Application Form for human, veterinary, and disinfectant drugs, as well as Clinical Trial Application/Attestation, lies with the drug manufacturer or sponsor.
FAQ
Q: What is the purpose of the Drug Submission Application Form?
A: The purpose of the Drug Submission Application Form is to apply for the approval of human, veterinary, or disinfectant drugs in Canada.
Q: What type of drugs can be submitted using this form?
A: This form can be used to submit applications for human, veterinary, or disinfectant drugs in Canada.
Q: Can this form be used for clinical trial applications?
A: Yes, this form can be used for clinical trial applications in Canada.
Q: What is the process for submitting a drug submission application?
A: The process for submitting a drug submission application involves completing the application form, providing supporting documentation, and paying the appropriate fees.
Q: Who can submit a drug submission application in Canada?
A: Any individual or organization that meets the eligibility criteria can submit a drug submission application in Canada.
Q: What is the purpose of the Clinical Trial Application/Attestation section?
A: The purpose of the Clinical Trial Application/Attestation section is to apply for the approval of clinical trials in Canada and provide information about the trial.
Q: Are there any specific requirements for clinical trial applications?
A: Yes, clinical trial applications must meet specific requirements outlined by Health Canada.
Q: What fees are associated with the submission of a drug submission application?
A: The fees associated with a drug submission application vary depending on the type of drug and the services required. It is recommended to refer to the current Fee Schedule published by Health Canada for detailed information.
Q: How long does it take to process a drug submission application?
A: The processing time for a drug submission application can vary depending on the complexity of the submission. It is recommended to refer to Health Canada's target review timelines for more information.