Drug Submission Application Form for: Human, Veterinary or Disinfectant Drugs and Clinical Trial Application / Attestation - Canada
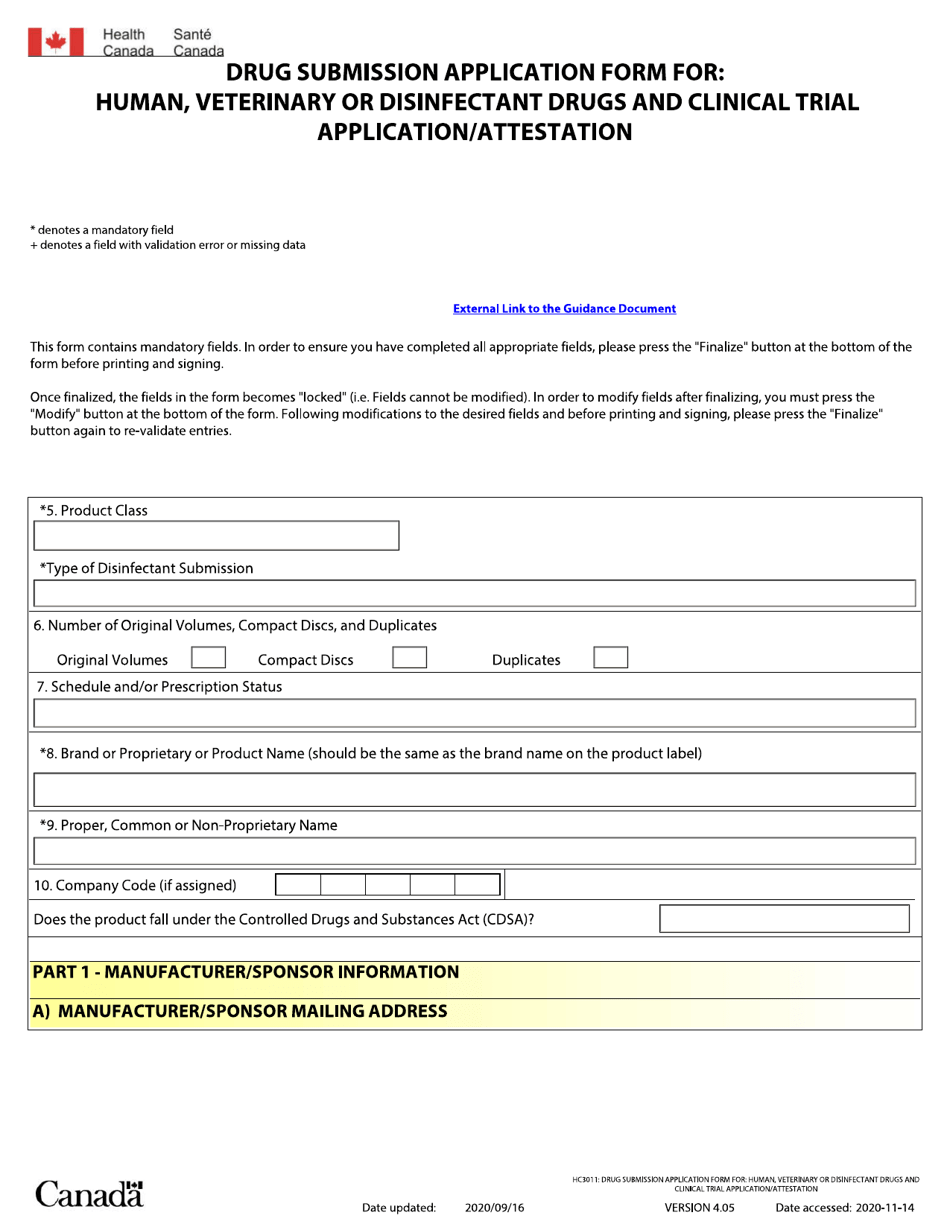
The Drug Submission Application Form for Human, Veterinary, or Disinfectant Drugs is used in Canada for submitting applications for the approval of drugs intended for use in humans, animals, or as disinfectants. The Clinical Trial Application/Attestation is used to apply for permission to conduct clinical trials in Canada.
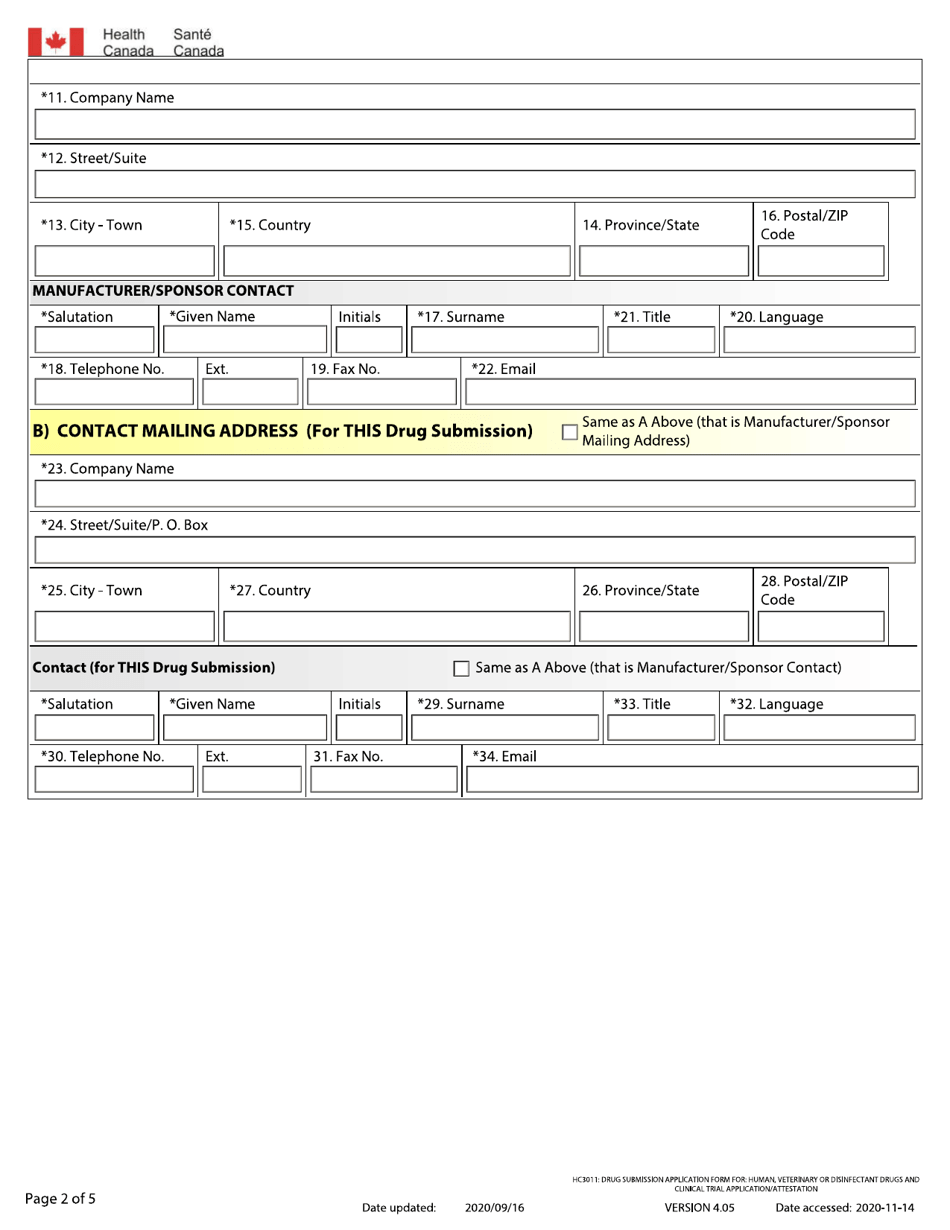
In Canada, the drug submission application form for human, veterinary, and disinfectant drugs, as well as clinical trial application/attestation, is filed by the drug manufacturer or sponsor.
Drug Submission Application Form for: Human, Veterinary or Disinfectant Drugs and Clinical Trial Application/Attestation - Canada - Frequently Asked Questions (FAQ)
Q: What is the purpose of the Drug Submission Application Form?
A: The purpose of the form is to apply for authorization to sell or distribute a human, veterinary, or disinfectant drug in Canada.
Q: Who can use the Drug Submission Application Form?
A: The form is used by applicants seeking approval for human, veterinary, or disinfectant drugs in Canada, or for conducting clinical trials.
Q: What information is required in the Drug Submission Application Form?
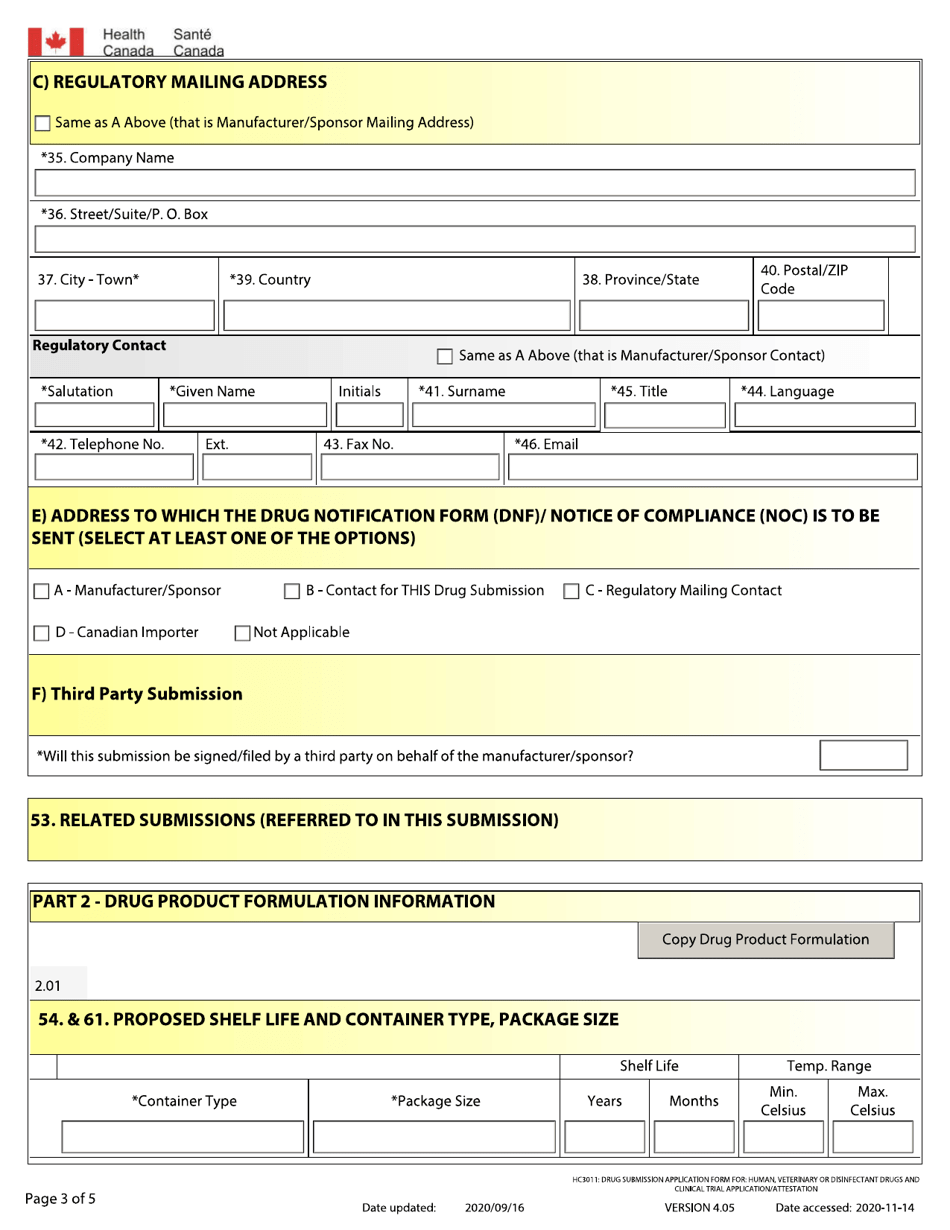
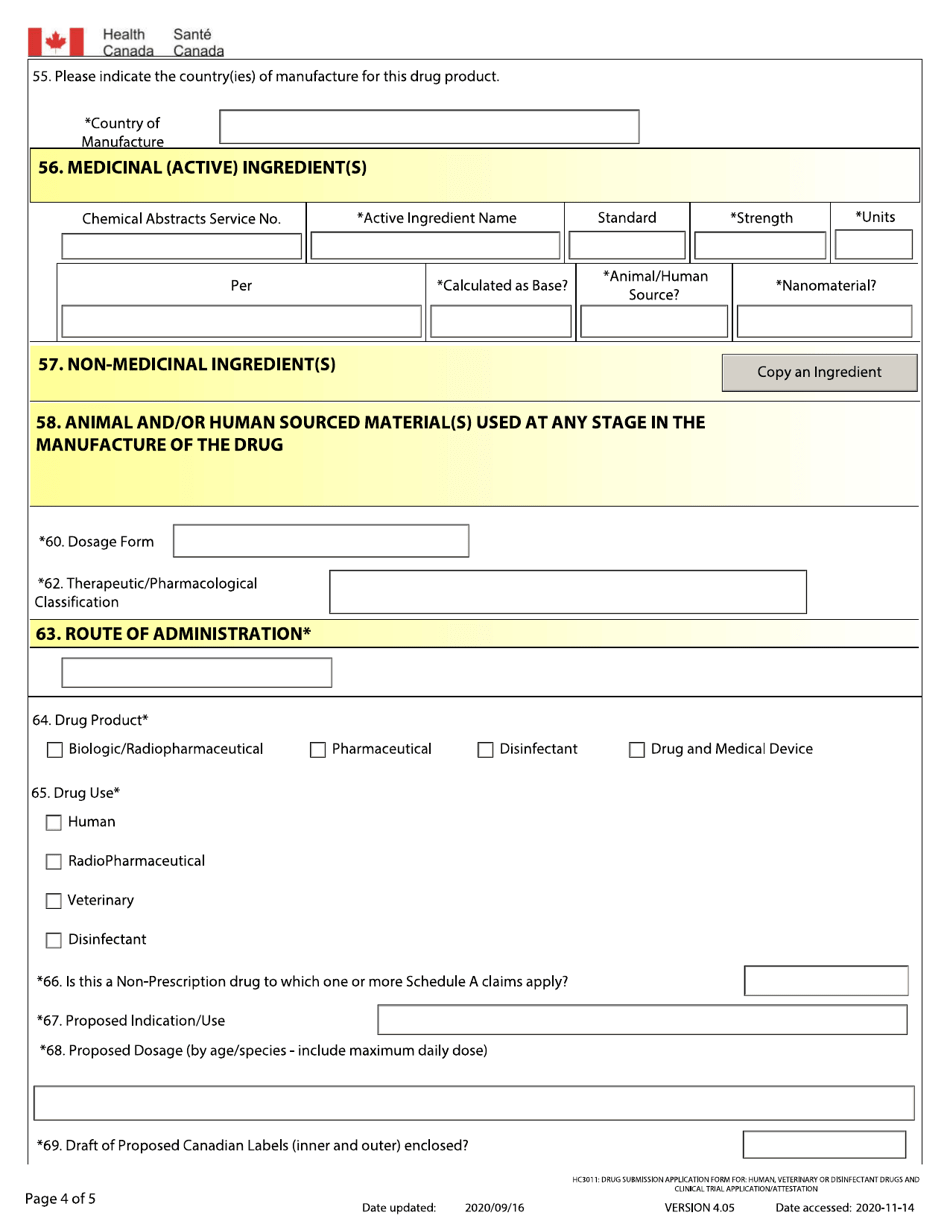
A: The form requires detailed information on the drug, including its formulation, indications, manufacturing process, and safety data.
Q: What should I include with the Drug Submission Application Form?
A: You should include supporting documents such as labelling, product monographs, and data on the drug's efficacy and safety.
Q: Are there any fees associated with the Drug Submission Application?
A: Yes, there are fees for submitting a drug submission application. The fee amount depends on the type of drug and the stage of the application process.
Q: What is a Clinical Trial Application/Attestation?
A: A Clinical Trial Application/Attestation is a separate form used to apply for authorization to conduct a clinical trial in Canada.