This version of the form is not currently in use and is provided for reference only. Download this version of
the document
for the current year.
Master File (Mf) Application Fee Form for Human Drugs - Canada
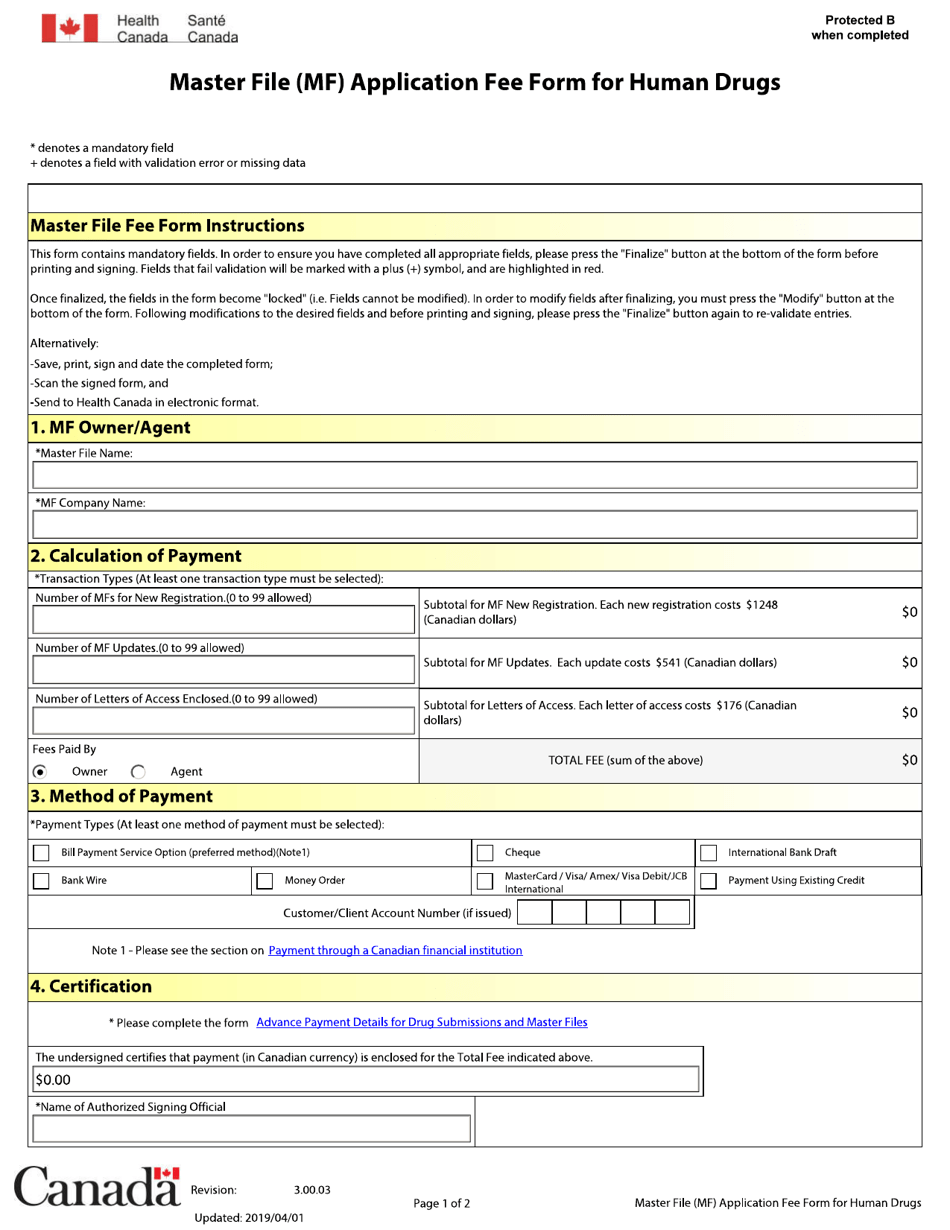
The Master File (MF) Application Fee Form for Human Drugs in Canada is used to submit an application for a master file for a human drug. The master file contains detailed information about the drug, such as its manufacturing process, quality control, and other relevant data. The purpose of this form is to provide the necessary information and pay the required fee to initiate the review process for a master file application for human drugs in Canada.
The Master File (MF) application fee form for human drugs in Canada is filed by the applicant or the sponsor of the drug.
FAQ
Q: What is the Master File (Mf) Application Fee Form for Human Drugs?
A: The Master File (Mf) Application Fee Form for Human Drugs is a form used in Canada for the application fee for the Master File (Mf) for human drugs.
Q: Who needs to fill out the Master File (Mf) Application Fee Form?
A: Companies in Canada that are applying for a Master File (Mf) for human drugs need to fill out the application fee form.
Q: What is the purpose of the Master File (Mf) for human drugs?
A: The Master File (Mf) for human drugs is a comprehensive document submitted by the drug manufacturer to provide detailed information about the quality, safety, and efficacy of the drug.
Q: How much is the application fee for the Master File (Mf) for human drugs?
A: The application fee for the Master File (Mf) for human drugs in Canada varies and is listed in the current fee schedule provided by the regulatory authorities.