Master File (Mf) Application Fee Form for Human Drugs - Canada
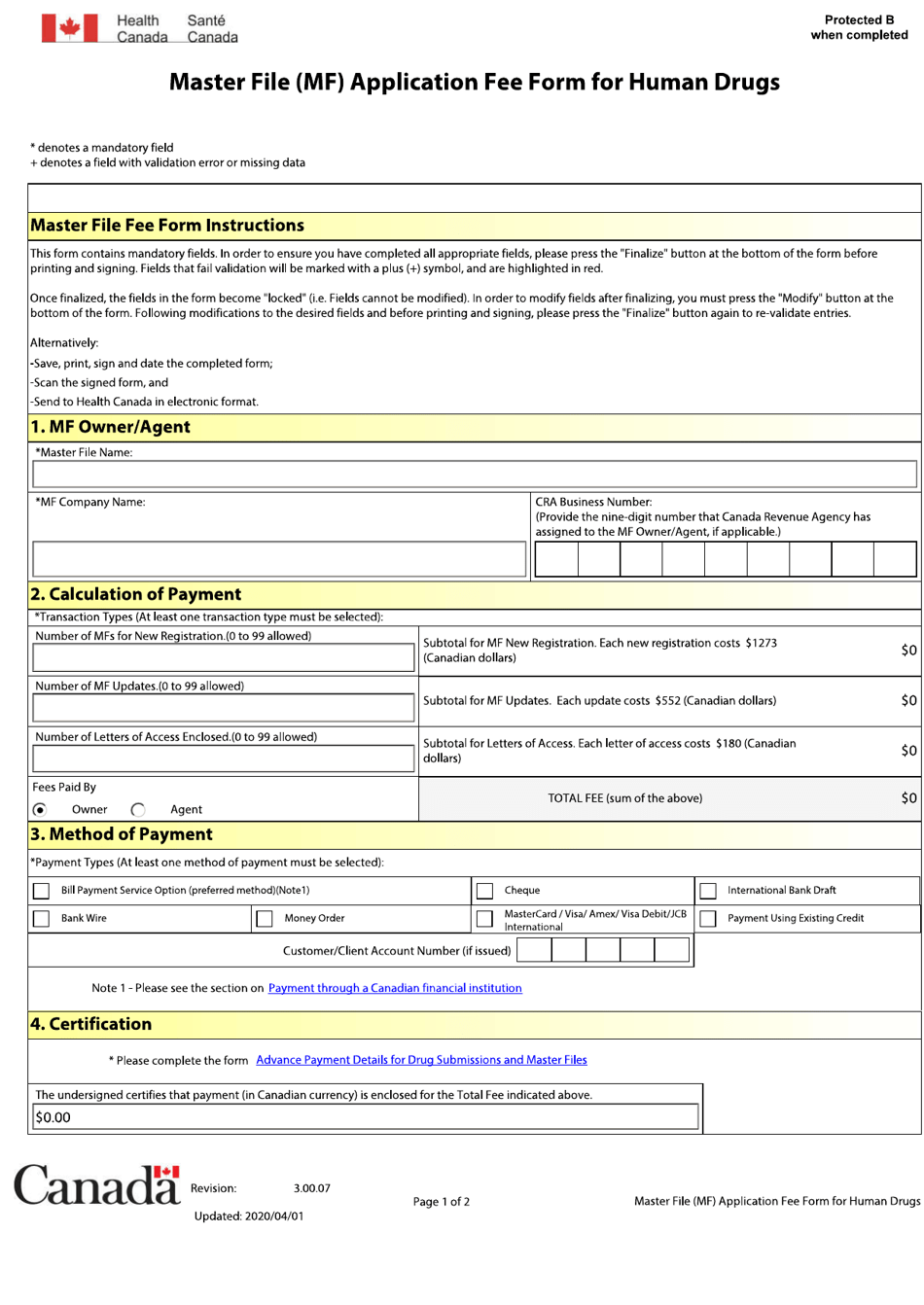
The Master File (MF) Application Fee Form for Human Drugs in Canada is used to pay the application fee for submitting a Master File. A Master File is a confidential document that provides detailed information about the manufacturing, quality control, and testing of a drug product. Pharmaceutical companies can submit a Master File to the regulatory authority to support the registration of their drug products. The application fee is required to process the submission of the Master File.
The Health Products and Food Branch (HPFB) of Health Canada is responsible for filing the Master File (MF) application fee form for human drugs in Canada.
Master File (Mf) Application Fee Form for Human Drugs - Canada - Frequently Asked Questions (FAQ)
Q: What is the Master File (Mf) Application Fee Form for Human Drugs?
A: The Master File (Mf) Application Fee Form is used for applying for a Master File (MF) related to human drugs in Canada.
Q: What is a Master File (Mf)?
A: A Master File (Mf) is a document submitted to Health Canada containing detailed information about the manufacturing, processing, and quality control of a drug substance or drug product.
Q: What is the purpose of the Master File (Mf)?
A: The purpose of the Master File (Mf) is to provide confidential, detailed information about the manufacturing and control of a drug substance or drug product that is used in support of a drug application.
Q: Who needs to submit a Master File (Mf) Application?
A: Manufacturers, packagers, and labellers who want to use a drug substance or drug product in a drug application need to submit a Master File (Mf) application.