Serious Adverse Drug Reaction Reporting Form for Hospitals - Canada
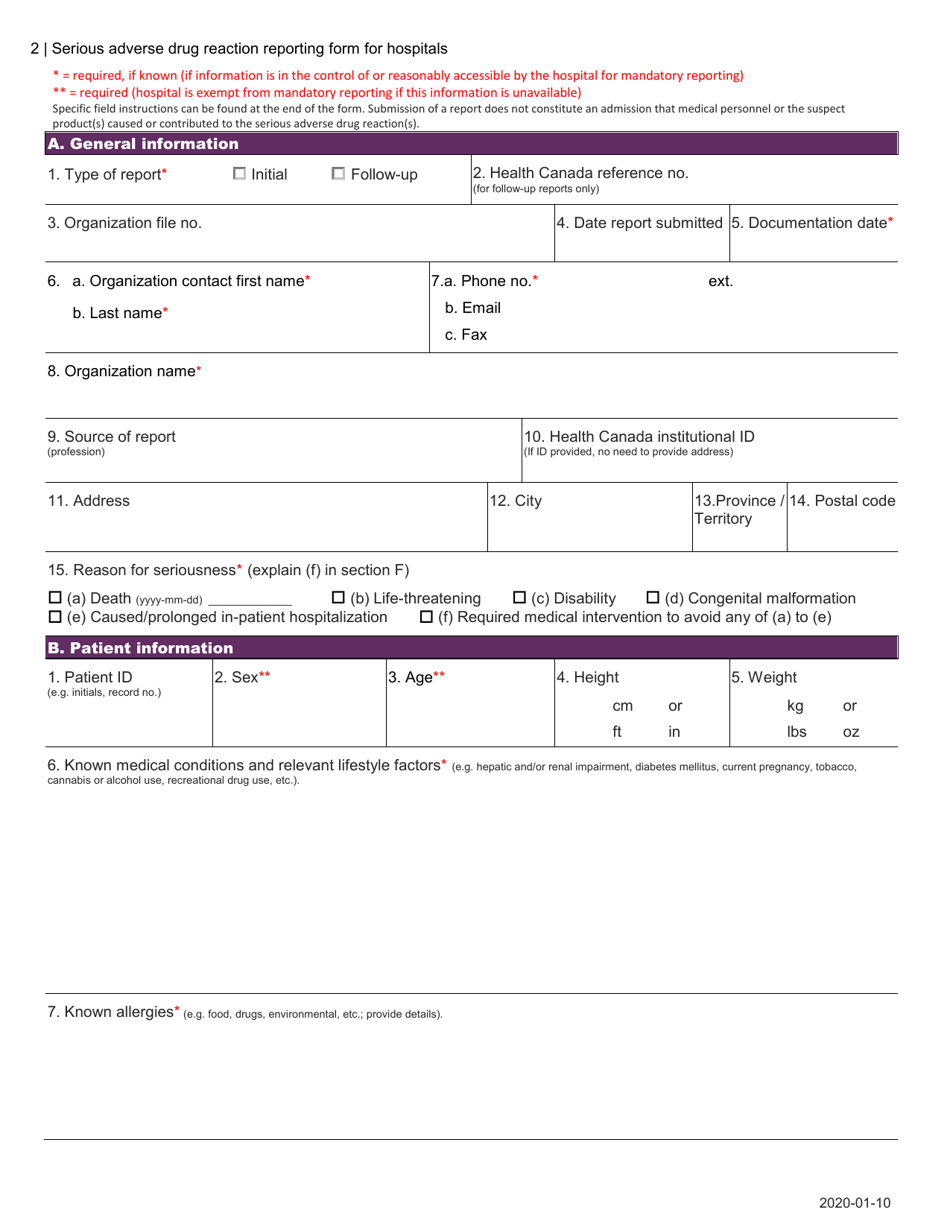
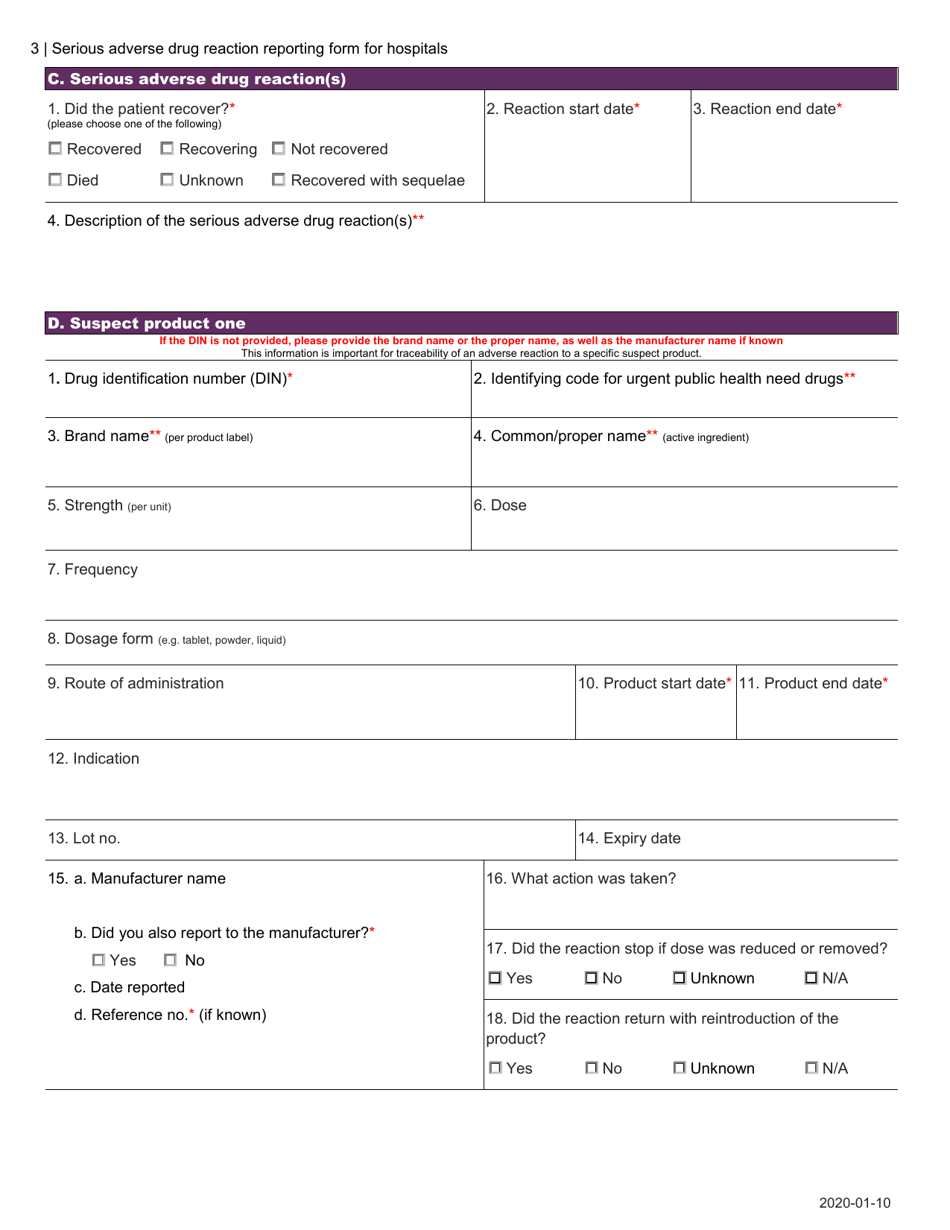
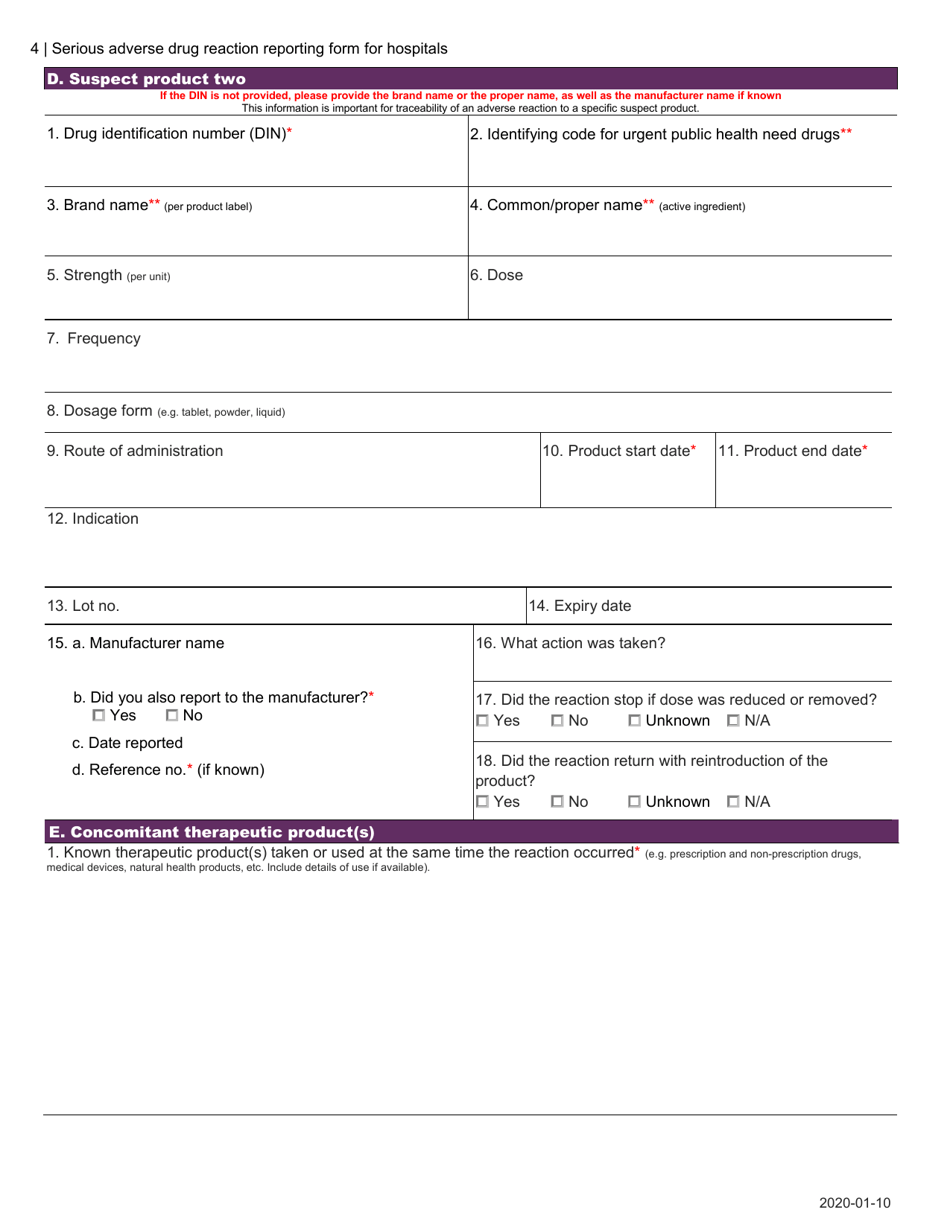
The Serious Adverse Drug Reaction Reporting Form for Hospitals in Canada is used to report any serious adverse reactions or events caused by the use of pharmaceutical drugs in hospital settings. This form helps to monitor and identify potential safety concerns related to medication usage.
In Canada, the Serious Adverse Drug Reaction Reporting Form for hospitals is typically filed by healthcare professionals or hospitals themselves.
FAQ
Q: What is the Serious Adverse Drug Reaction (ADR) Reporting Form for Hospitals in Canada?
A: The Serious Adverse Drug Reaction Reporting Form for Hospitals in Canada is a form used to report any serious adverse drug reactions that occur in a hospital setting.
Q: Who is responsible for reporting serious adverse drug reactions in hospitals?
A: Hospitals in Canada are responsible for reporting serious adverse drug reactions.
Q: What is considered a serious adverse drug reaction?
A: A serious adverse drug reaction is any unexpected or severe reaction to a medication that requires hospitalization, prolongs an existing hospital stay, results in permanent damage or disability, or poses a threat to life.
Q: Why is it important to report serious adverse drug reactions?
A: Reporting serious adverse drug reactions is important to ensure patient safety, identify potential medication risks, and improve the overall quality of healthcare.
Q: How can hospitals report serious adverse drug reactions?
A: Hospitals can report serious adverse drug reactions using the Serious Adverse Drug Reaction Reporting Form for Hospitals, which can be submitted electronically or by mail.
Q: What information is required when reporting a serious adverse drug reaction?
A: When reporting a serious adverse drug reaction, hospitals are required to provide details about the patient, the medication involved, the reaction experienced, and any contributing factors.
Q: Are hospitals required to report all adverse drug reactions, or only serious ones?
A: Hospitals are only required to report serious adverse drug reactions. However, it is encouraged to report all adverse drug reactions to help monitor medication safety.
Q: Who receives the reports of serious adverse drug reactions in hospitals?
A: The reports of serious adverse drug reactions in hospitals are received by Health Canada, the national health regulatory agency.
Q: What happens after a serious adverse drug reaction is reported?
A: After a serious adverse drug reaction is reported, Health Canada reviews the information, investigates the incident if necessary, and takes appropriate actions to address medication safety concerns.