This version of the form is not currently in use and is provided for reference only. Download this version of
the document
for the current year.
Labels and Packages Certification Form for Non-prescription Drugs - Canada
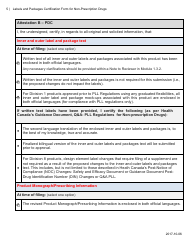
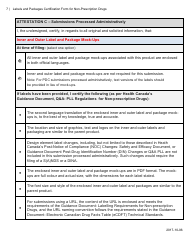
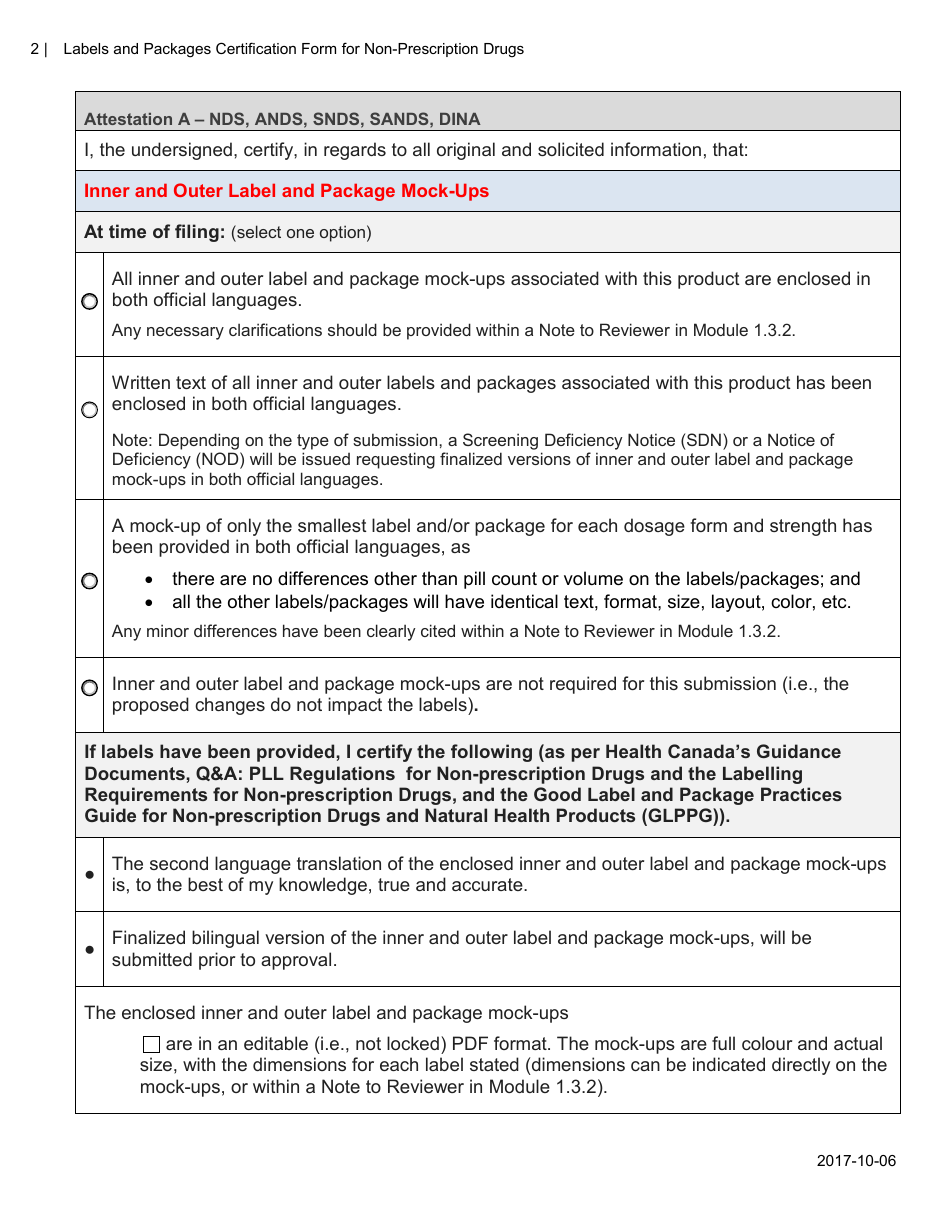
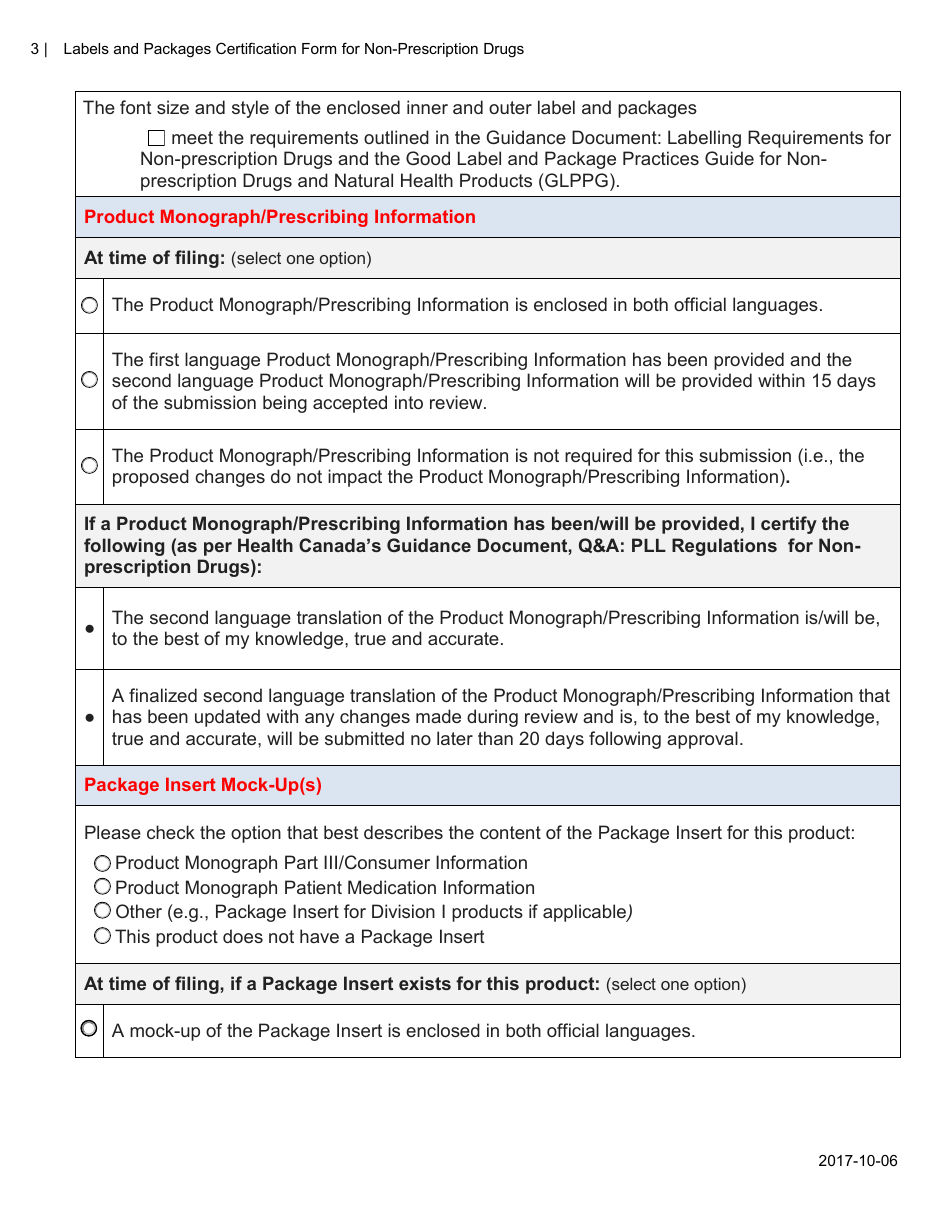
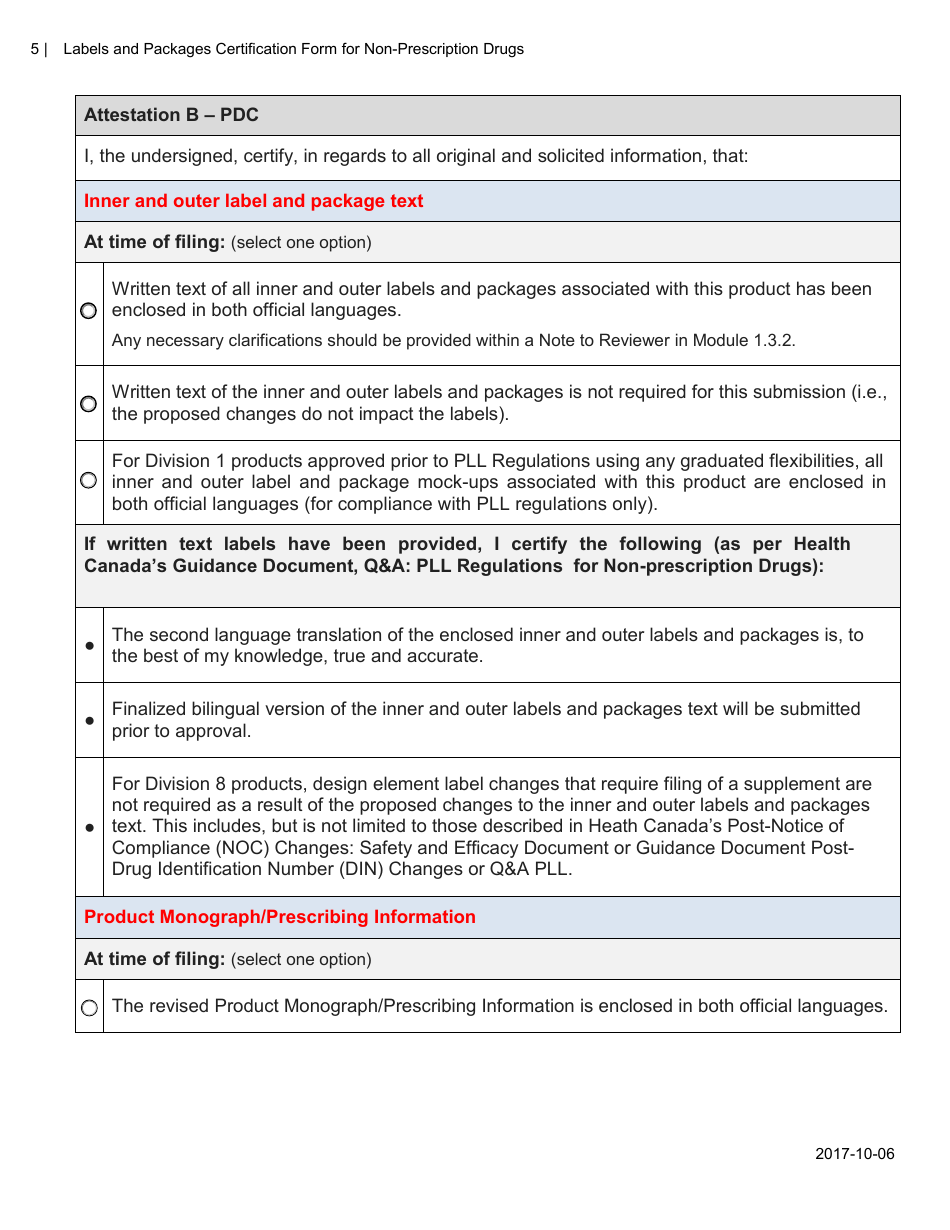
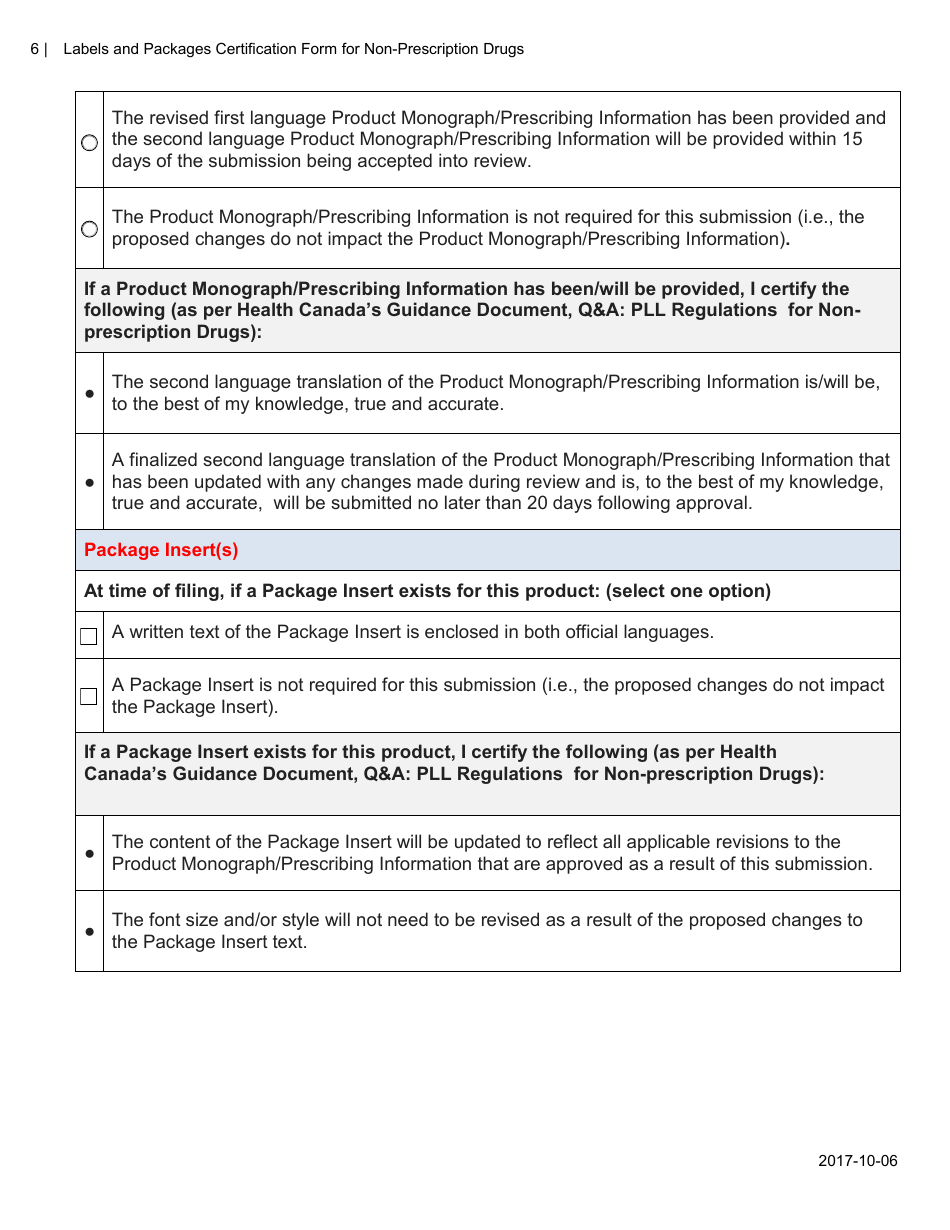
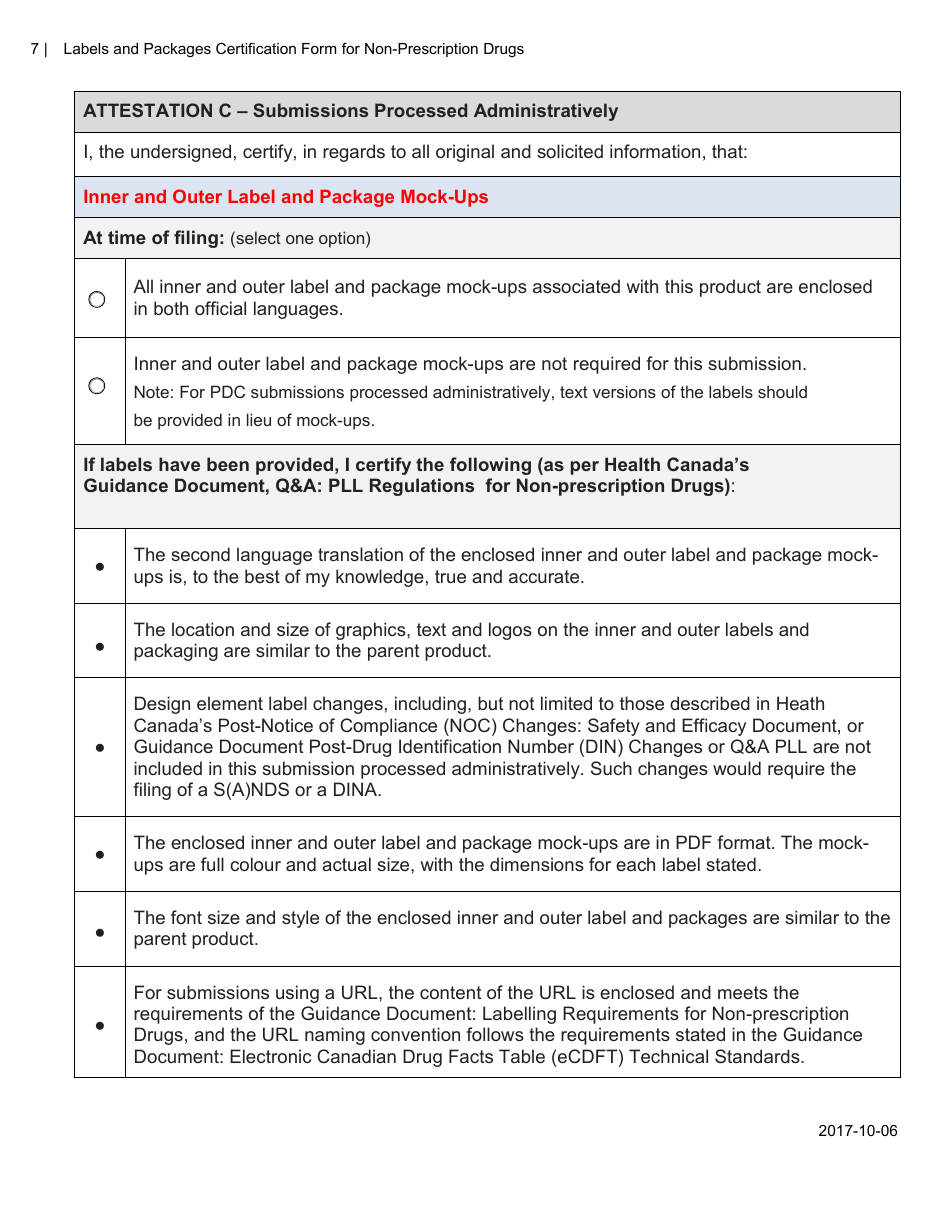
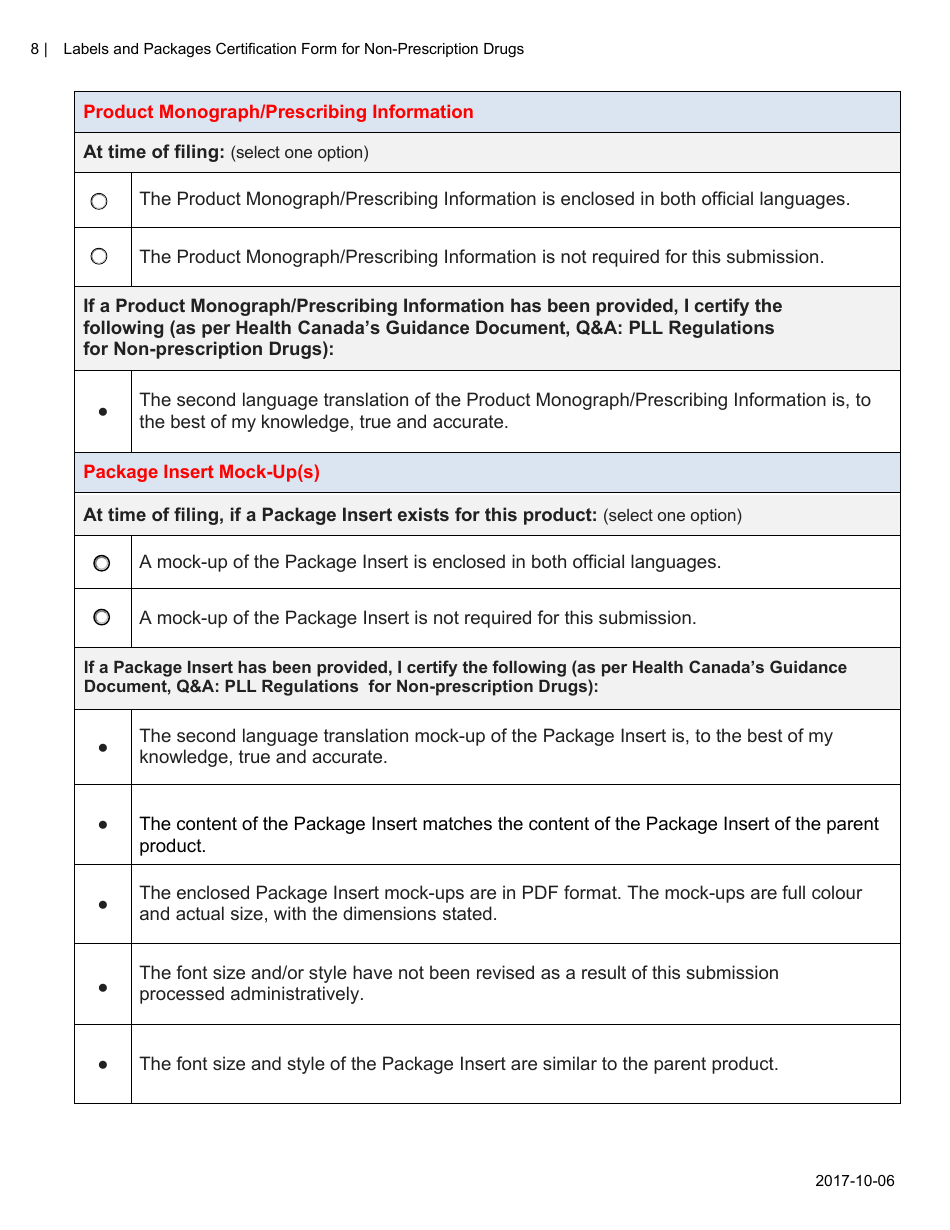
The Labels and Packages Certification Form for Non-prescription Drugs in Canada is used to certify that the labels and packages of non-prescription drugs meet the regulatory requirements set by Health Canada. It ensures that the information provided on the labels and packages is accurate and compliant with the applicable rules and regulations.
The manufacturer or importer of non-prescription drugs in Canada is responsible for filing the Labels and Packages Certification Form.
FAQ
Q: What is the Labels and Packages Certification Form for Non-prescription Drugs?
A: The Labels and Packages Certification Form is a document used in Canada to certify the accuracy and completeness of labeling and packaging for non-prescription drugs.
Q: Who needs to complete the Labels and Packages Certification Form?
A: Manufacturers and importers of non-prescription drugs in Canada need to complete the Labels and Packages Certification Form.
Q: What is the purpose of the Labels and Packages Certification Form?
A: The purpose of the form is to ensure that the labeling and packaging of non-prescription drugs comply with applicable regulations and provide accurate information to consumers.
Q: What information is included in the Labels and Packages Certification Form?
A: The form includes information such as the product's name, dosage form, active ingredients, indications, warnings, and directions for use.