Labels and Packages Certification Form for Non-prescription Drugs - Canada
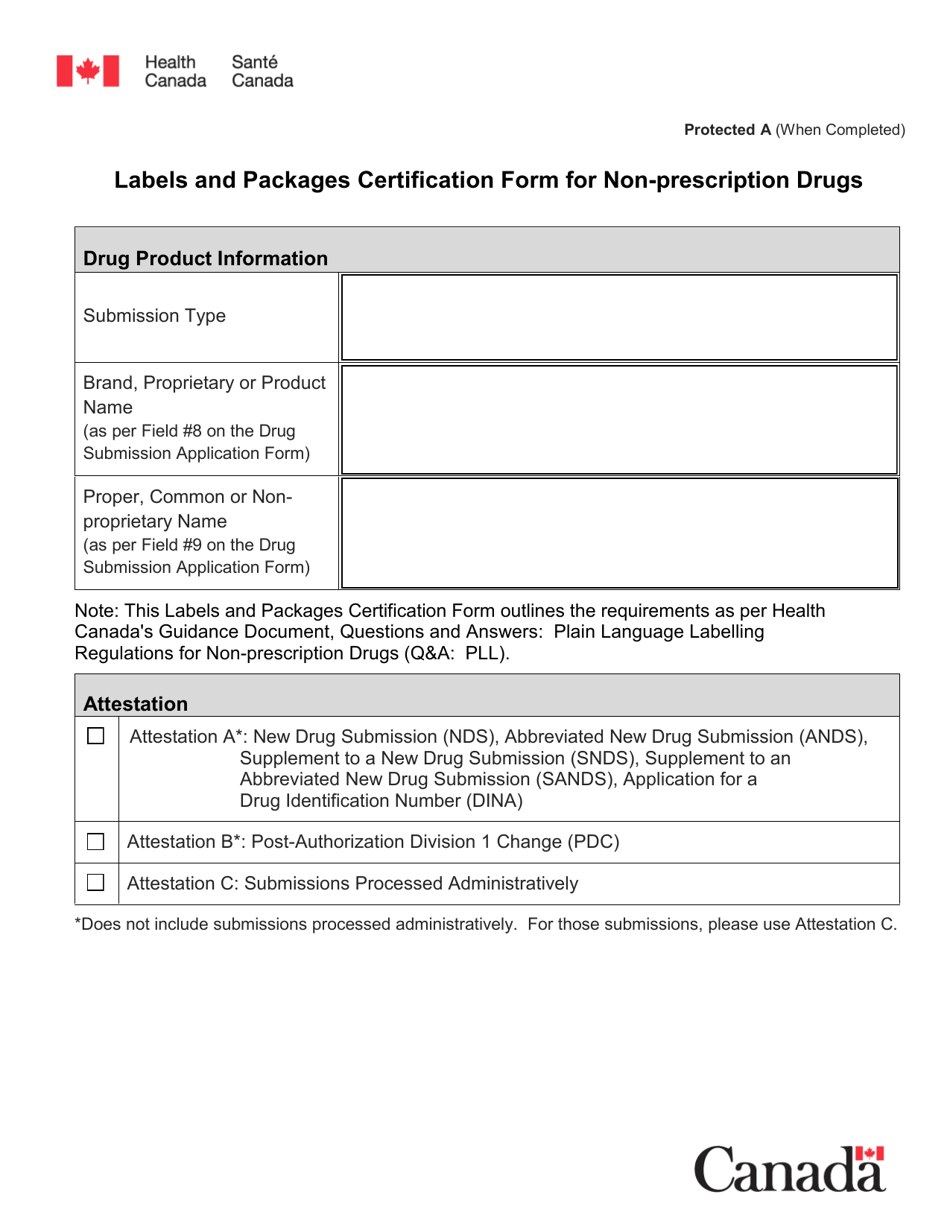
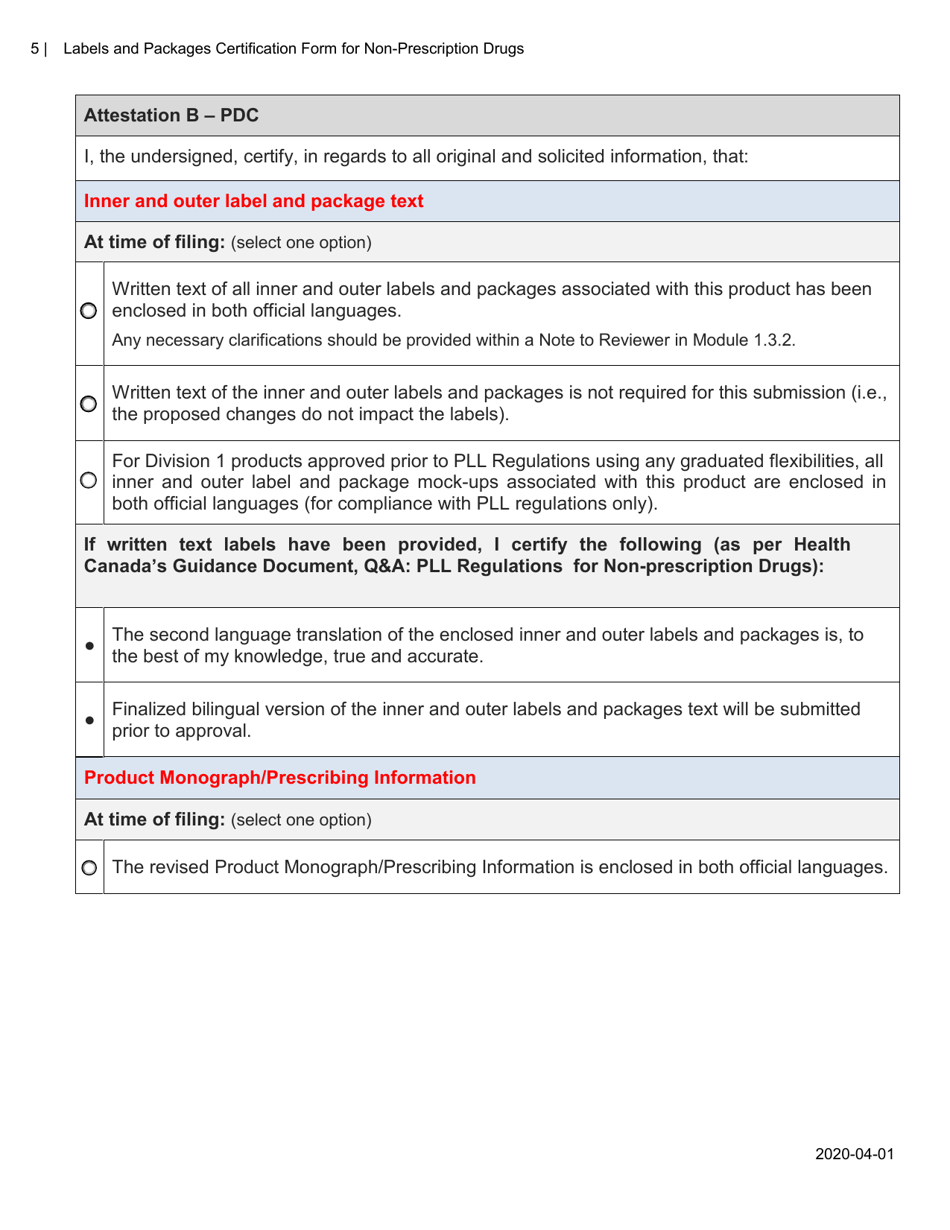
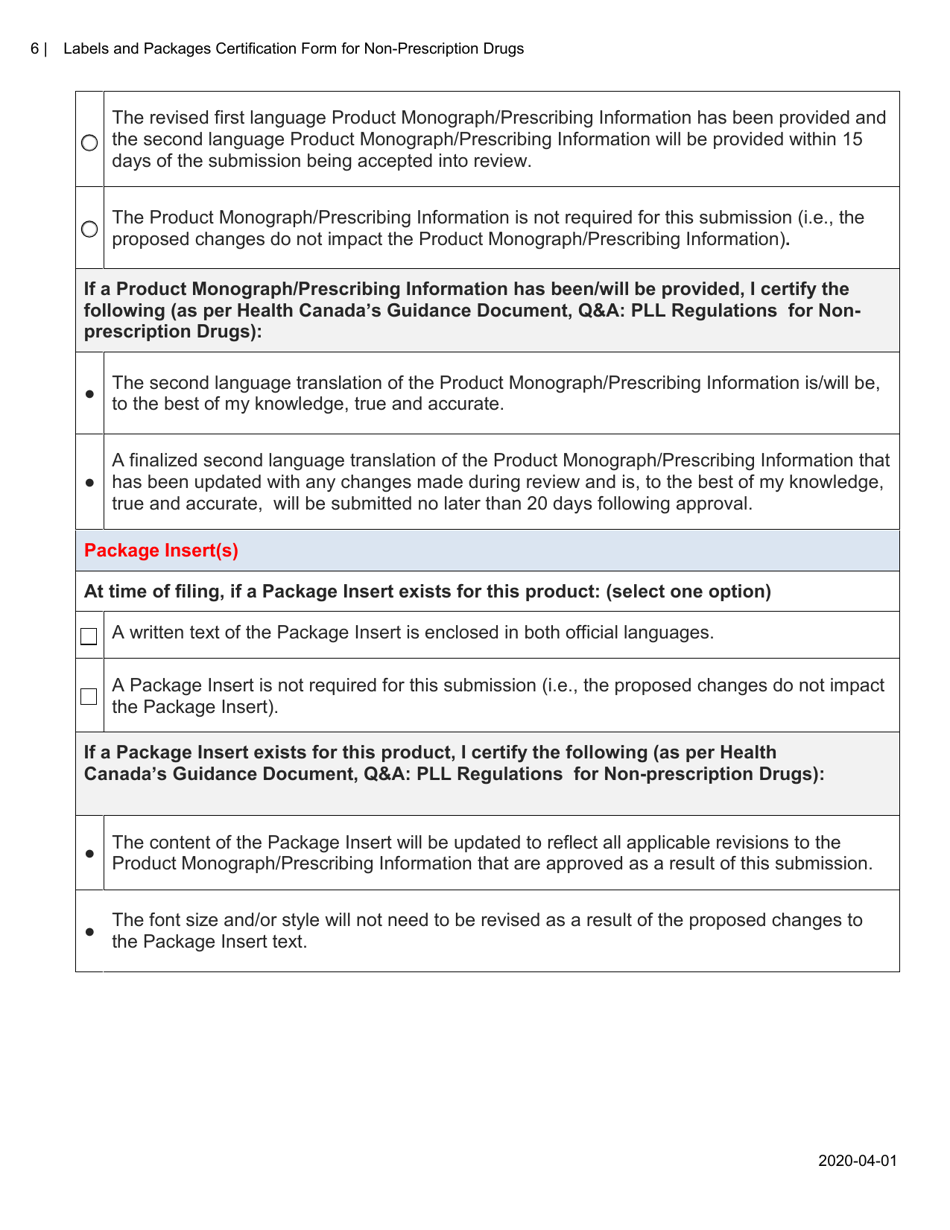
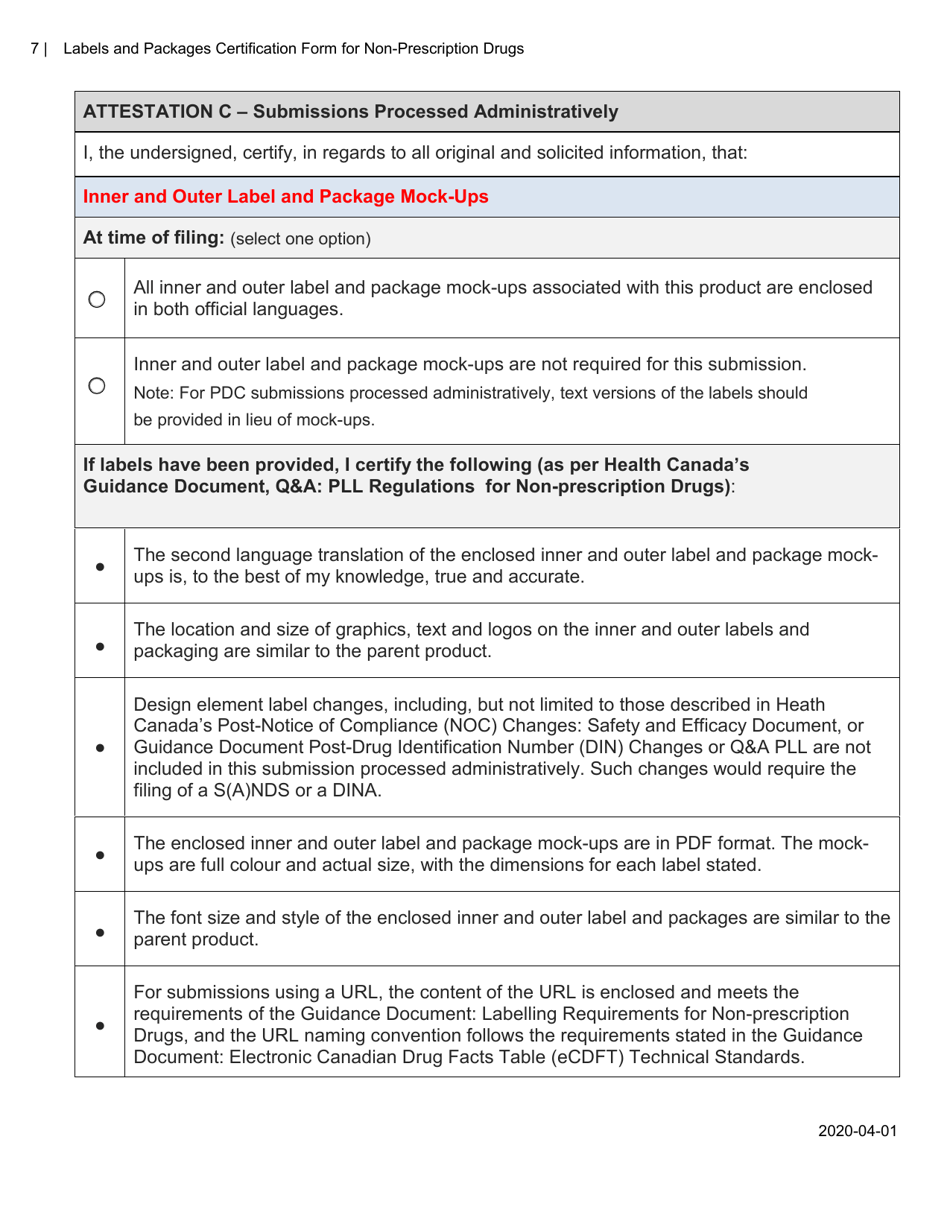
The Labels and Packages Certification Form for Non-prescription Drugs in Canada is used to certify that the labels and packages of non-prescription drugs meet the regulatory requirements set by Health Canada. It ensures that the information on the labels and packages of these drugs is accurate, clear, and provides necessary instructions for safe use.
The manufacturer or distributor of non-prescription drugs in Canada typically files the Labels and Packages Certification Form.
Labels and Packages Certification Form for Non-prescription Drugs - Canada - Frequently Asked Questions (FAQ)
Q: What is the Labels and Packages Certification Form for Non-prescription Drugs in Canada?
A: The Labels and Packages Certification Form is a document used to ensure that the labels and packages of non-prescription drugs in Canada meet the regulatory requirements.
Q: Who needs to fill out the Labels and Packages Certification Form for Non-prescription Drugs in Canada?
A: Manufacturers or importers of non-prescription drugs in Canada need to fill out this form.
Q: What is the purpose of the Labels and Packages Certification Form for Non-prescription Drugs in Canada?
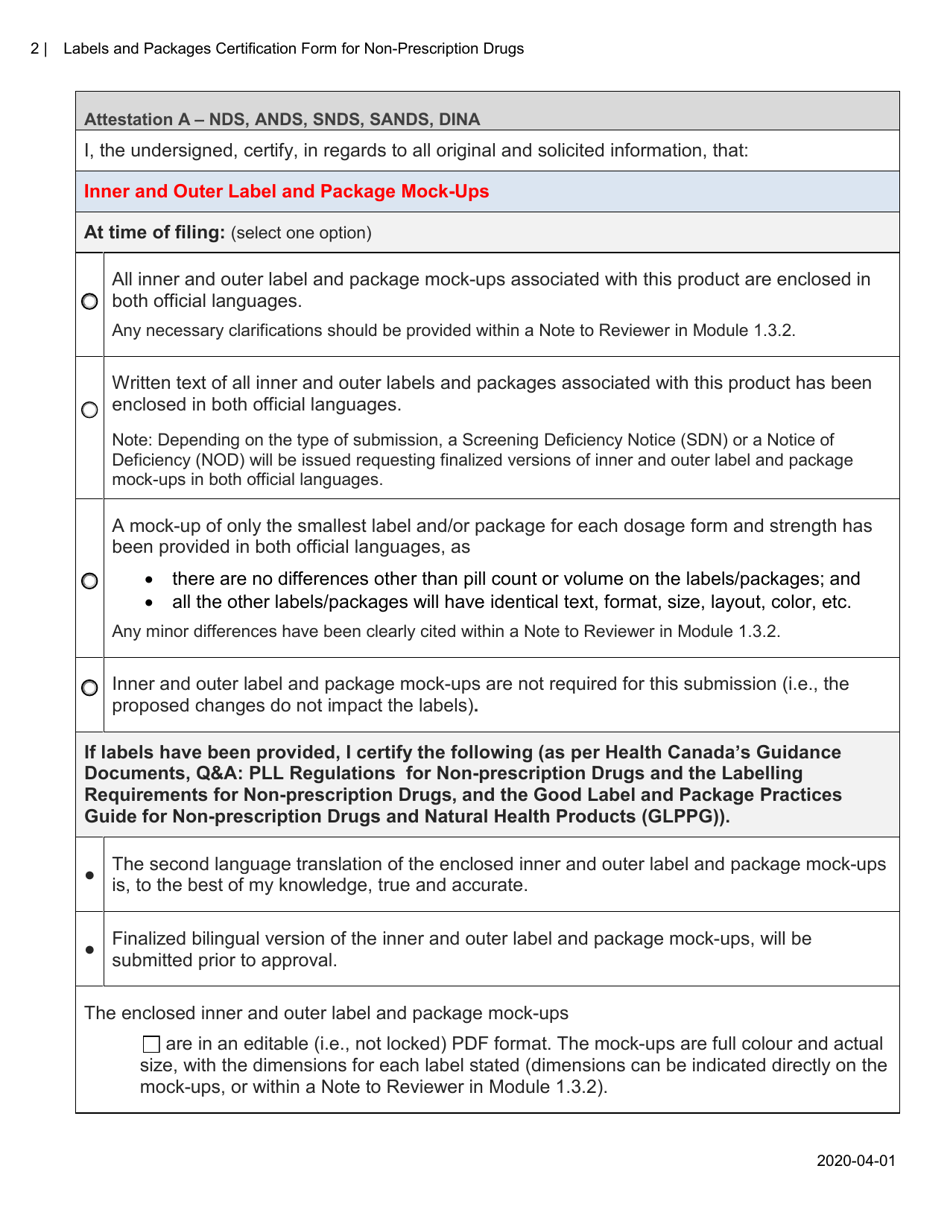
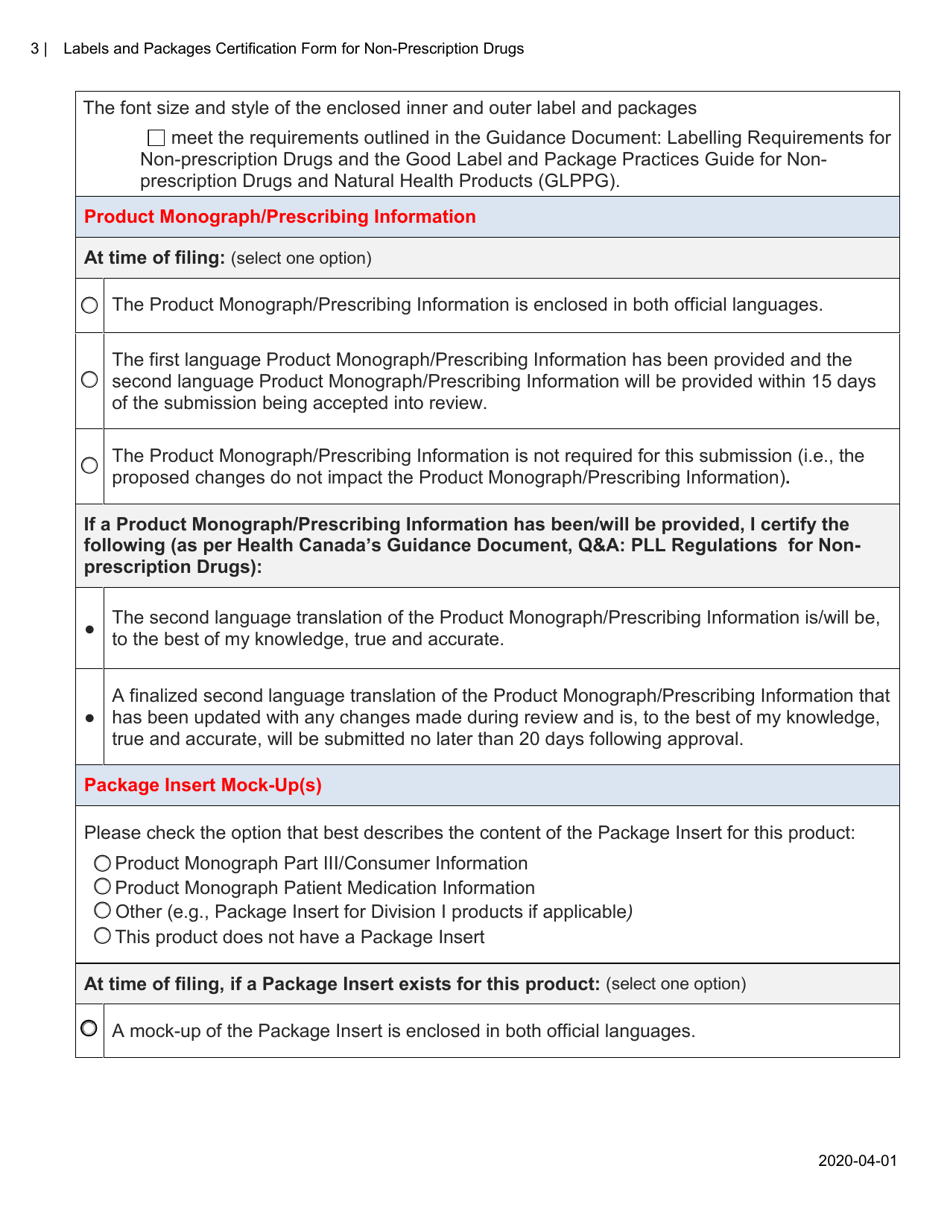
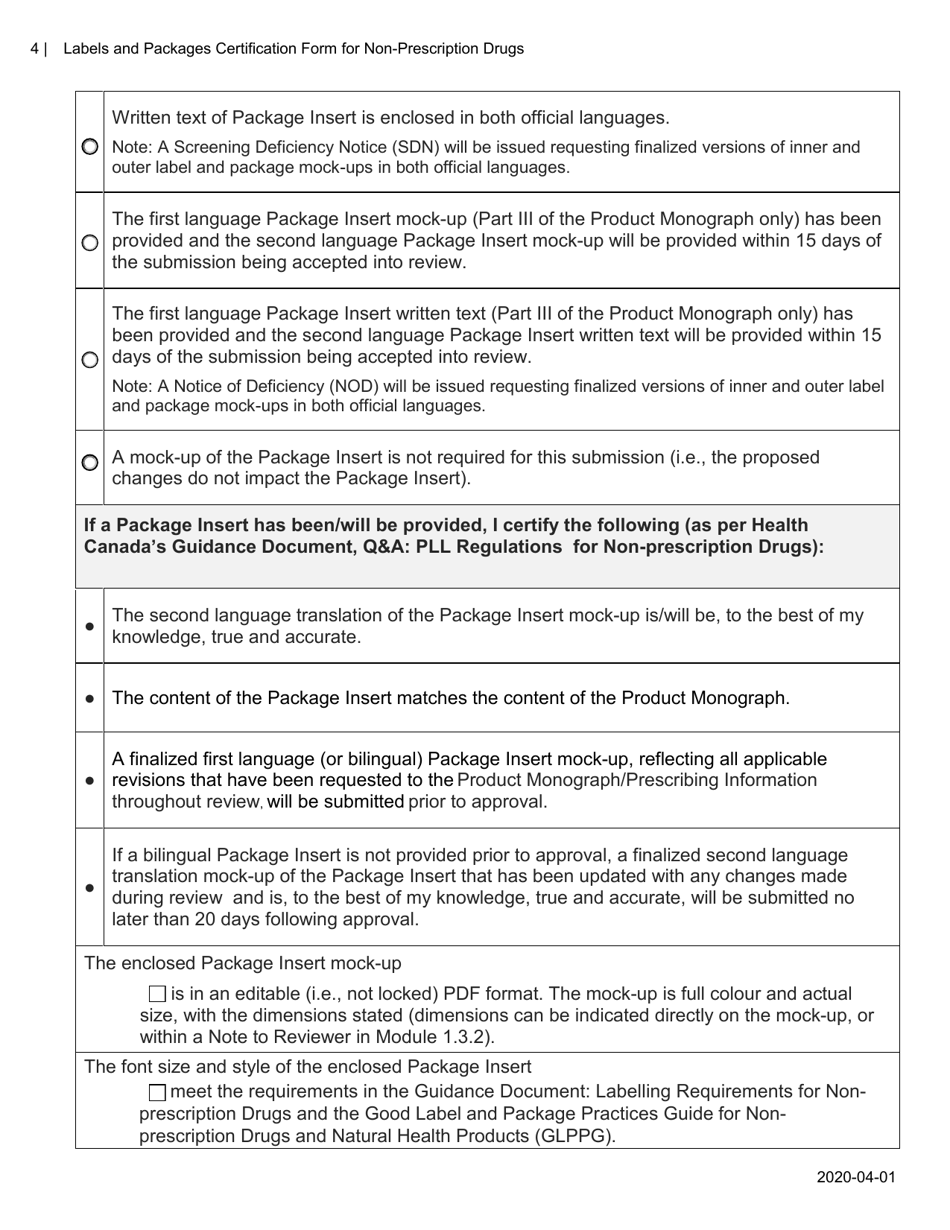
A: The purpose of this form is to verify that the labels and packages of non-prescription drugs comply with Canadian regulations, including proper ingredient listing, health warning statements, and accurate product information.
Q: What information is required in the Labels and Packages Certification Form for Non-prescription Drugs in Canada?
A: The form requires information such as the product name, active ingredients, lot number, expiry date, and approved health claims.
Q: Are there any fees associated with submitting the Labels and Packages Certification Form for Non-prescription Drugs in Canada?
A: Yes, there are fees associated with the submission of this form. The exact fees depend on the type and quantity of products being certified.
Q: What happens after submitting the Labels and Packages Certification Form for Non-prescription Drugs in Canada?
A: After submission, Health Canada will review the form and determine if the labels and packages comply with the regulations. If approved, a Certificate of Compliance will be issued.
Q: Is the Labels and Packages Certification Form for Non-prescription Drugs in Canada mandatory?
A: Yes, it is mandatory for manufacturers or importers of non-prescription drugs in Canada to fill out this form and obtain a Certificate of Compliance before selling their products.
Q: What should I do if there are changes to the labels or packages of my non-prescription drug after obtaining the Certificate of Compliance?
A: If there are any changes to the labels or packages, a new Labels and Packages Certification Form must be submitted to Health Canada for review and approval.