U.S. Department of Health and Human Services Forms
Documents:
429
This document is used for agreeing to assign a claim upon request.
This form is used for reporting cases of Multisystem Inflammatory Syndrome associated with Covid-19.
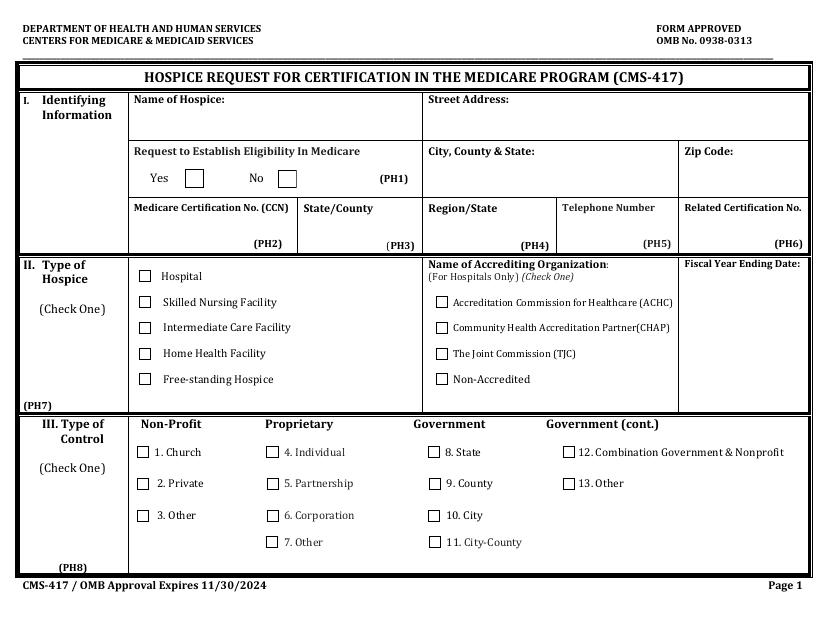
This Form is used for hospices to request certification in the Medicare Program. It is required for hospices to participate in the program and provide services to Medicare beneficiaries.
This form is used for verifying clinic data for the Rural Health Clinic Program.
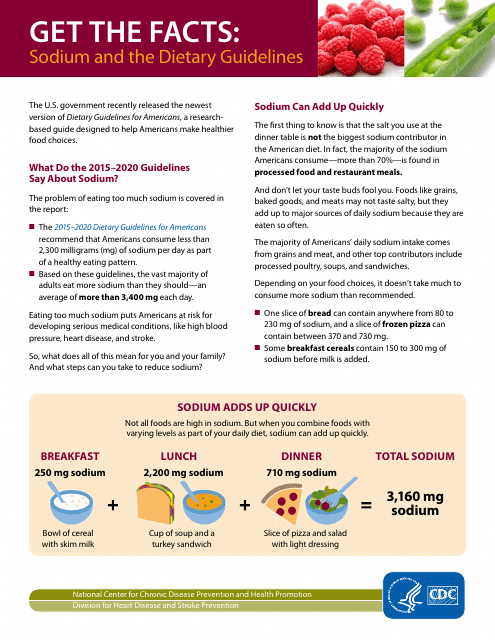
This document provides information about sodium and its relationship to the Dietary Guidelines.
This form is used for obtaining consent from individuals who wish to undergo sterilization procedures.
This document outlines the plan for implementing the Low Income Household Water Assistance Program (LIHWAP) in Florida. It includes details on how the program will be funded and administered to provide financial assistance to low-income households for their water needs.
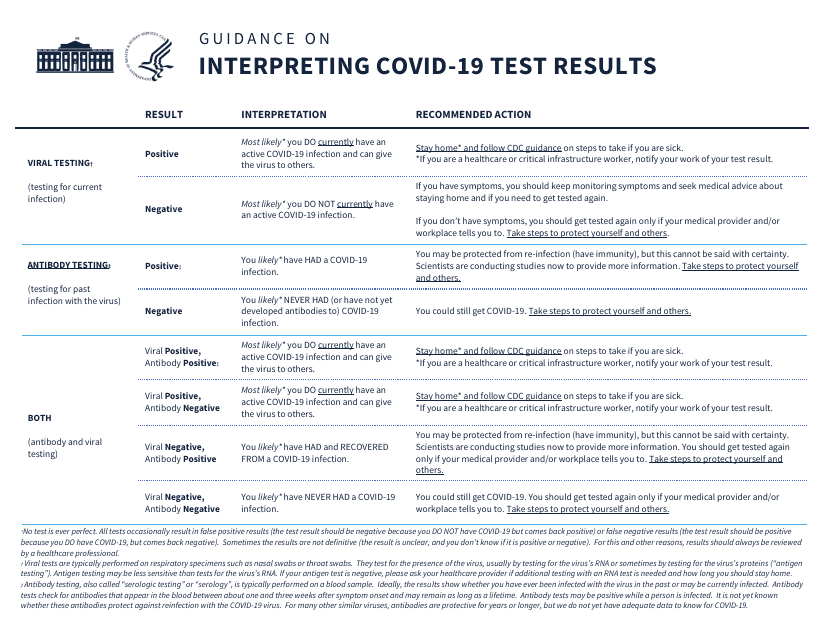
This document provides guidance on how to interpret the results of Covid-19 tests. It helps individuals understand what their test results mean in relation to their health and potential risk of transmitting the virus.
This form is used for registering a multistate employer for new hire reporting. It ensures compliance with state requirements for reporting new employee hires.
This type of document is used for eligible ordering and referring physicians and non-physician practitioners to register for Medicare enrollment.
Medicare beneficiaries may use this form to authorize individuals or organizations they trust to examine their medical records and learn their protected health information.
This form is used for completing a pre-vaccination checklist for individuals receiving the Covid-19 vaccine.
This Form is used for applying for a special enrollment period in Medicare Part A and Part B in cases of exceptional conditions.
This form is used for creating a checklist of essential items needed in a pet disaster kit. It includes items such as food, water, medications, and identification tags.
This form is used for recording concurrence and approval of chapters in the NIH Manual.
This Form is used for implementing enhanced barrier precautions in skilled nursing facilities and nursing homes. It helps to improve the safety measures against infections.
This Form is used for auditing and reporting on single-service containers and closures used by milk and milk product manufacturers.
This form is used for conducting investigations on farms regarding additional water sources that are not included in the initial questionnaire.
This form is used for reporting the results of inspections conducted at bakeries by the FDA.
This form is used for applying for a variance from the FDA regulations regarding laser light shows, displays, or devices. It allows individuals or organizations to request permission to deviate from the specific requirements outlined in 21 CFR 1040.11(C) in relation to laser safety.
This form is used for reporting accidental radiation occurrences to the FDA.
This form is used for submitting abbreviated reports on the radiation safety of non-medical ultrasonic products to the FDA. It helps ensure that these products meet safety standards and do not pose any health risks to users.
This document is used for submitting abbreviated reports on radiation safety for microwave products other than microwave ovens to the FDA.
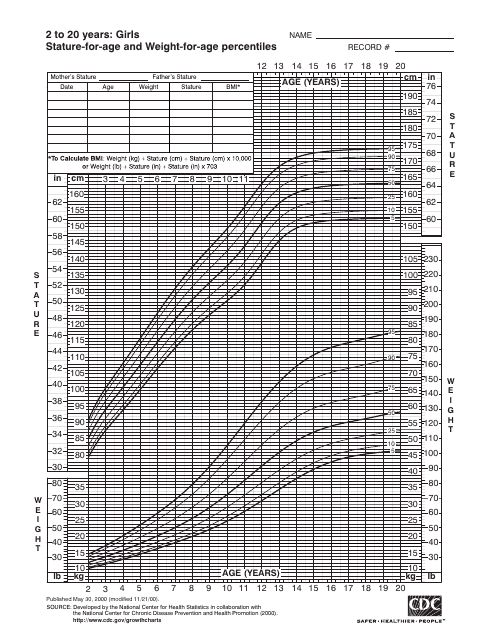
This document provides stature-for-age and weight-for-age charts for girls between the ages of 2 to 20 years. It helps track the growth and development of girls and can be used by parents, caregivers, and healthcare professionals.