U.S. Department of Health and Human Services Forms
Documents:
429
This Form is used for reporting cases of Tickborne Rickettsial Disease.
This form is used for reporting cases of Babesiosis, a tick-borne disease, to the Centers for Disease Control and Prevention (CDC). It helps track and monitor the incidence of Babesiosis in the United States.
This document is a Varicella Surveillance Worksheet used for tracking and monitoring cases of varicella (chickenpox) infection.
This form is used for investigating cases of cryptosporidiosis related to Cryptonet.
This Form is used for reporting and investigating cases of plague, a dangerous infectious disease.
This document is used for tracking and documenting information related to suspected cases of polio.
This document is used for tracking and reporting cases of pertussis, a contagious respiratory infection commonly known as whooping cough. It helps healthcare providers gather information necessary for surveillance and prevention efforts.
This document is for collecting data related to mumps cases as part of surveillance efforts.
This form is used for reporting cases of Brucellosis, a bacterial infection that can be transmitted from animals to humans. It collects important information about the infected individual and helps healthcare professionals track and monitor the disease.
This form is used for reporting cases of Q Fever, a bacterial infection that can cause flu-like symptoms and is transmitted to humans from animals.
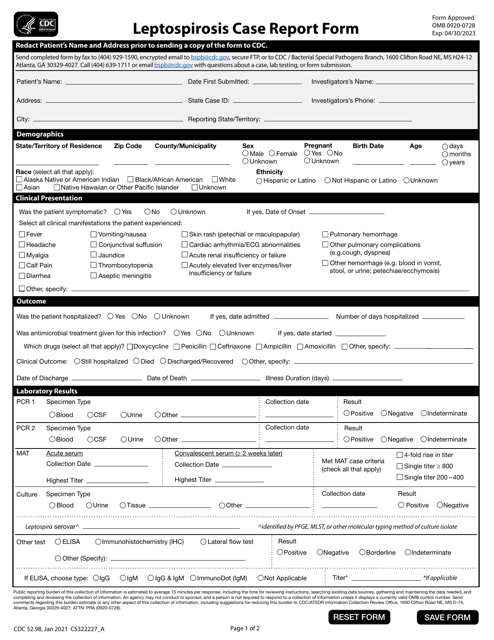
This form is used for reporting cases of leptospirosis, a bacterial infection spread through contact with contaminated water.
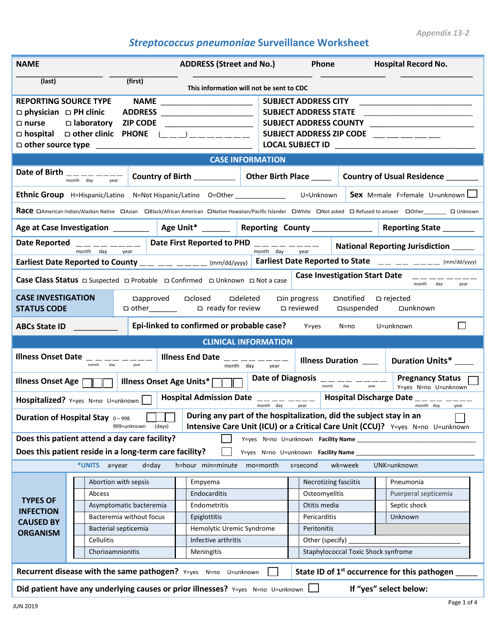
This Form is used for tracking cases of Streptococcus Pneumoniae.
This form is used for reporting and documenting cases of human infection with the 2019 Novel Coronavirus.
Este tipo de formulario se utiliza como parte de las medidas de prevención del CDC (Centros para el Control y la Prevención de Enfermedades) para combatir la Listeria, una bacteria que puede causar enfermedades transmitidas por los alimentos.
This form is used for non-construction programs to provide assurances to the federal government. It ensures that the program will comply with all applicable laws and regulations.
This form is used for transmitting periodic reports and promotional material for new animal drugs to the FDA.
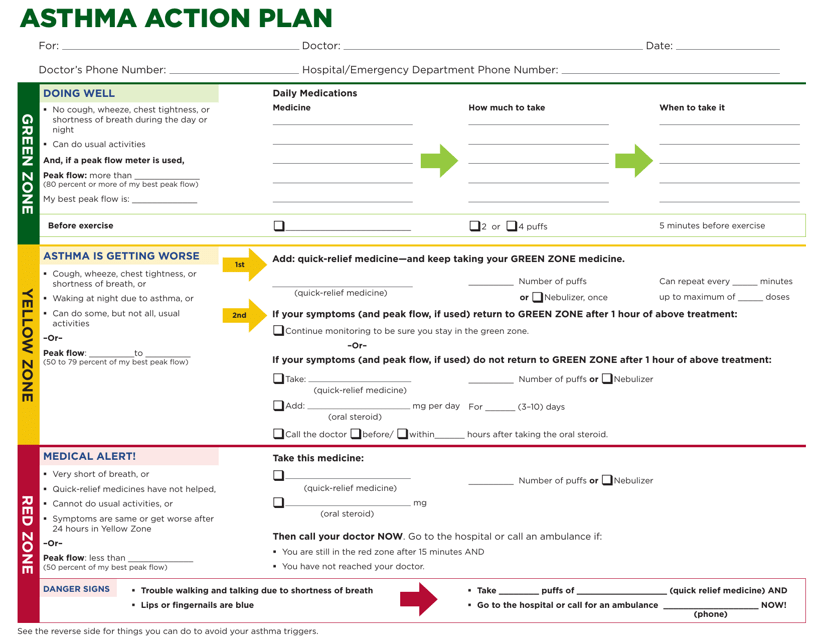
This document is a personalized plan that provides instructions on how to manage asthma symptoms and what steps to take in different situations. It helps individuals with asthma to better understand and control their condition.
This form is used for assessing the capacity of ports to handle the impacts of Covid-19. It is in French.
This form is used for conducting a rapid assessment of the capacity of a point of entry (POE) to handle Covid-19 cases. The form is in Russian.
This form is used for assessing the capacity of ports during the Covid-19 pandemic. It is specifically designed for Arabic speakers.
This document provides a pocket-sized card with information on the outbreak of Hepatitis A, including its symptoms, transmission, and prevention methods.
This type of document is a pocket card that provides information and reminders about getting vaccinated for Hepatitis A.
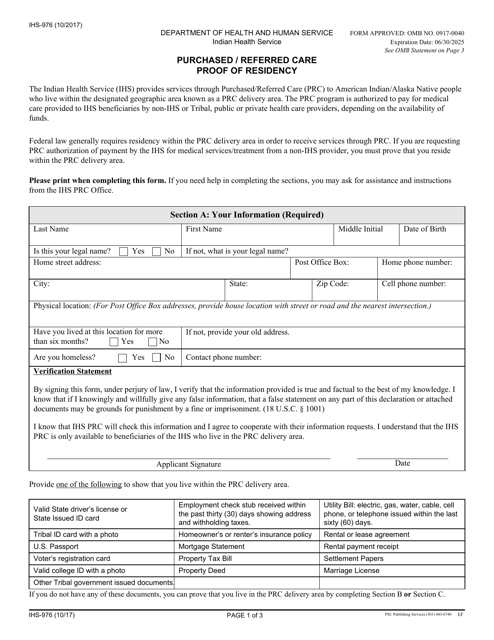
This form is used for providing proof of residency for Native American beneficiaries seeking purchased/referred care services.
This form is used for appointing a representative for individuals who prefer large print format.
This Form is used for appointing a representative with large print for better accessibility.
This Form is used for organizations to certify their ability to electronically exchange files with the Centers for Medicare & Medicaid Services (CMS).
This form is used for transmitting gifts to the National Institutes of Health (NIH). It serves as a record of the gift and provides information about the donor and the gift itself.
This document is a Gift Acceptance Evaluation Checklist that is used by the National Institutes of Health (NIH) in the United States. It is used to assess and evaluate gifts that are offered to the NIH.
This form is used for providing outstanding loan information.
This form is used for tracking the medication regimen for latent tuberculosis (TB) infection. It covers a 3-month period and includes daily doses of Isoniazid and Rifampin.
This form is used for transferring control of infection between different healthcare facilities.