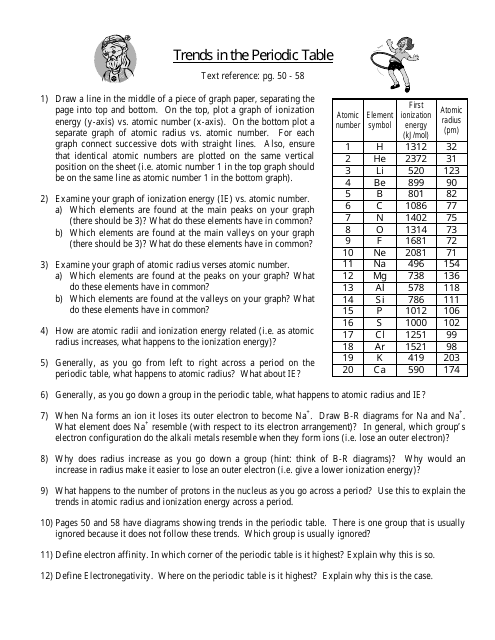
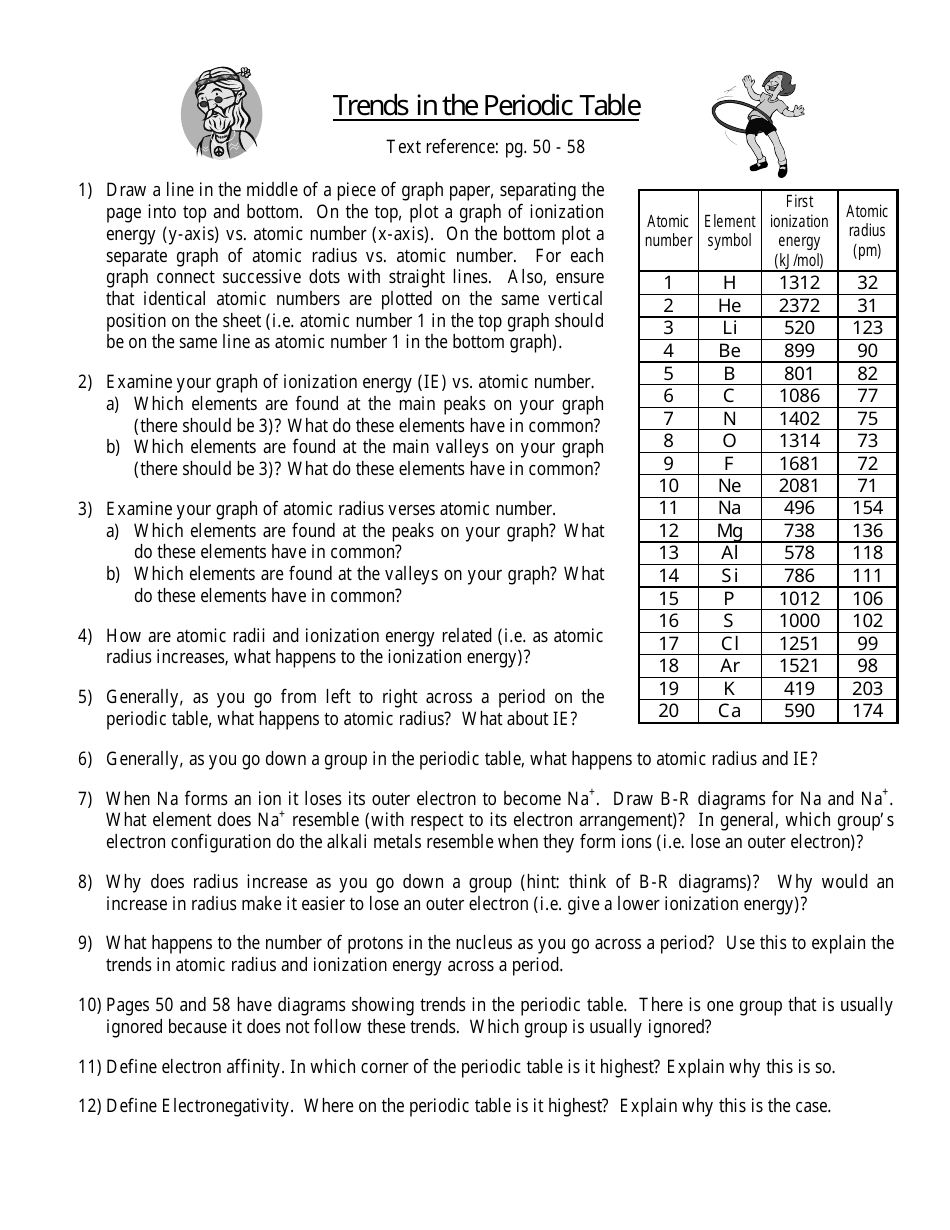
Periodic Table Trends Worksheet
Periodic Table Trends Worksheet is a tool used to study and understand the trends in the properties of elements in the periodic table. It helps in identifying patterns in elements' atomic size, ionization potential, electronegativity, and other characteristics.
The periodic table trends worksheet is typically filed by students or teachers in educational settings.
FAQ
Q: What are periodic table trends?
A: Periodic table trends refer to the patterns or relationships that can be observed among the elements on the periodic table.
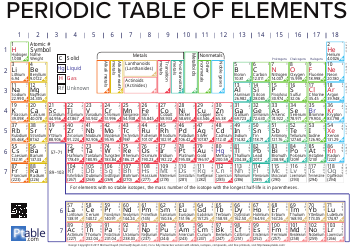
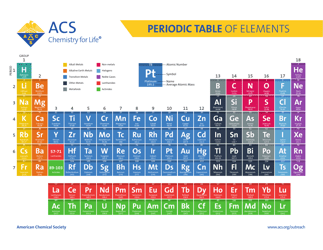
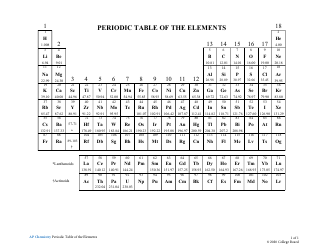
Q: What is the periodic table?
A: The periodic table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties.
Q: What are some examples of periodic table trends?
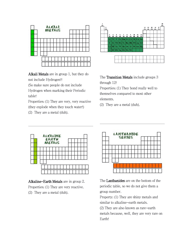
A: Examples of periodic table trends include atomic radius, ionization energy, electronegativity, and metallic character.
Q: What is atomic radius?
A: Atomic radius is the size of an atom, which generally decreases across a period and increases down a group on the periodic table.
Q: What is ionization energy?
A: Ionization energy is the energy required to remove an electron from an atom, which generally increases across a period and decreases down a group on the periodic table.
Q: What is electronegativity?
A: Electronegativity is the measure of an atom's ability to attract electrons in a chemical bond, which generally increases across a period and decreases down a group on the periodic table.
Q: What is metallic character?
A: Metallic character refers to the ability of an element to exhibit metallic properties, such as malleability, conductivity, and luster, which generally decreases across a period and increases down a group on the periodic table.