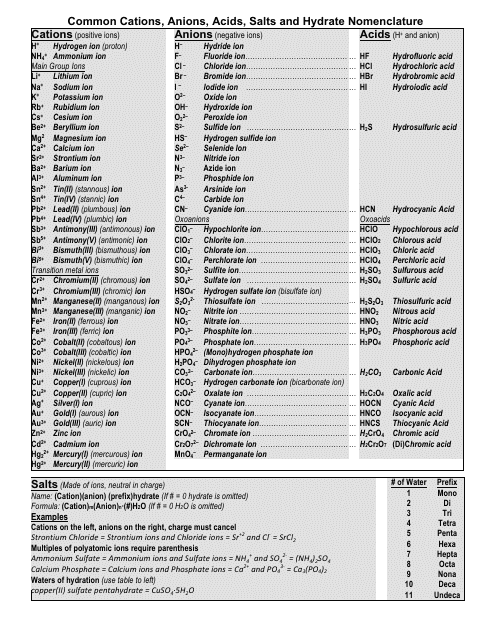
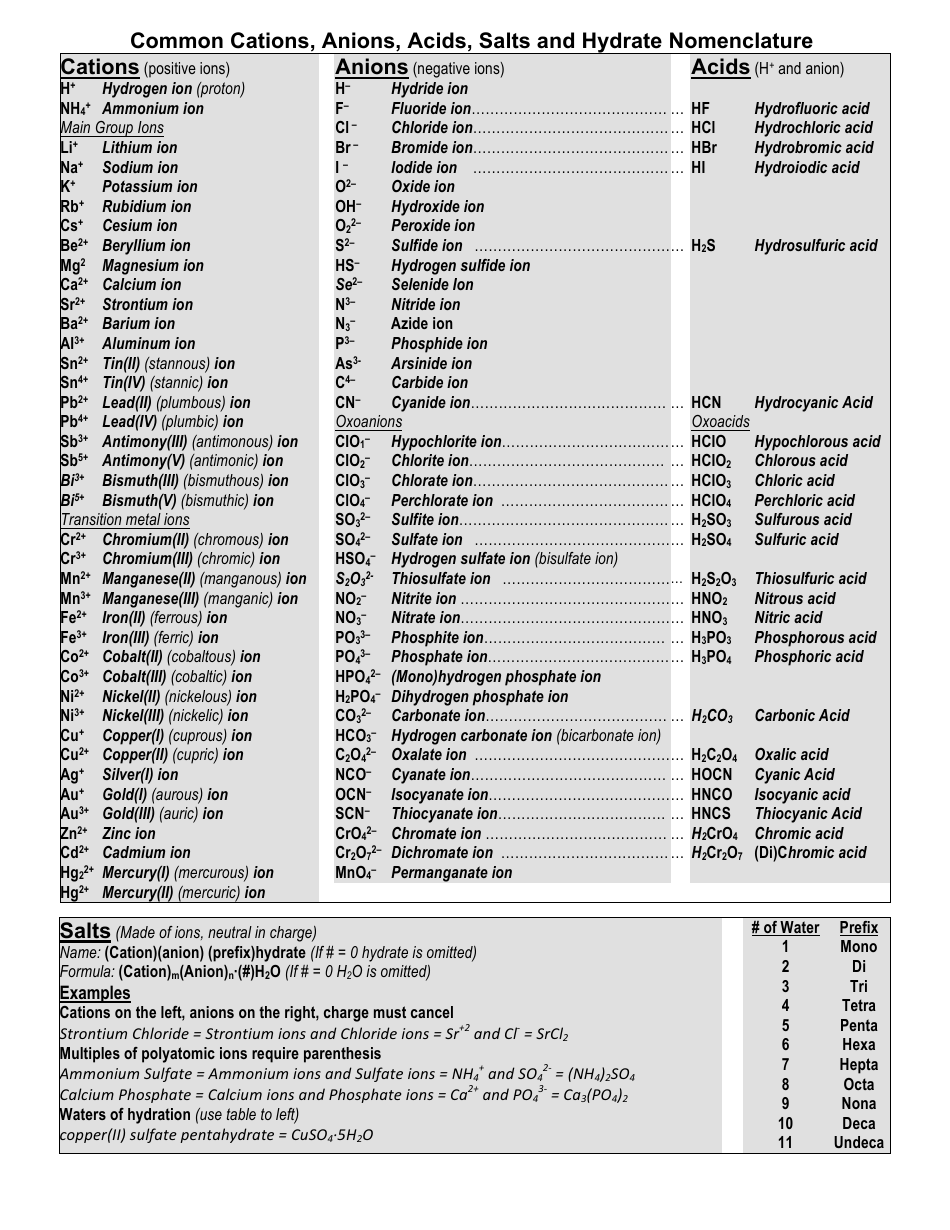
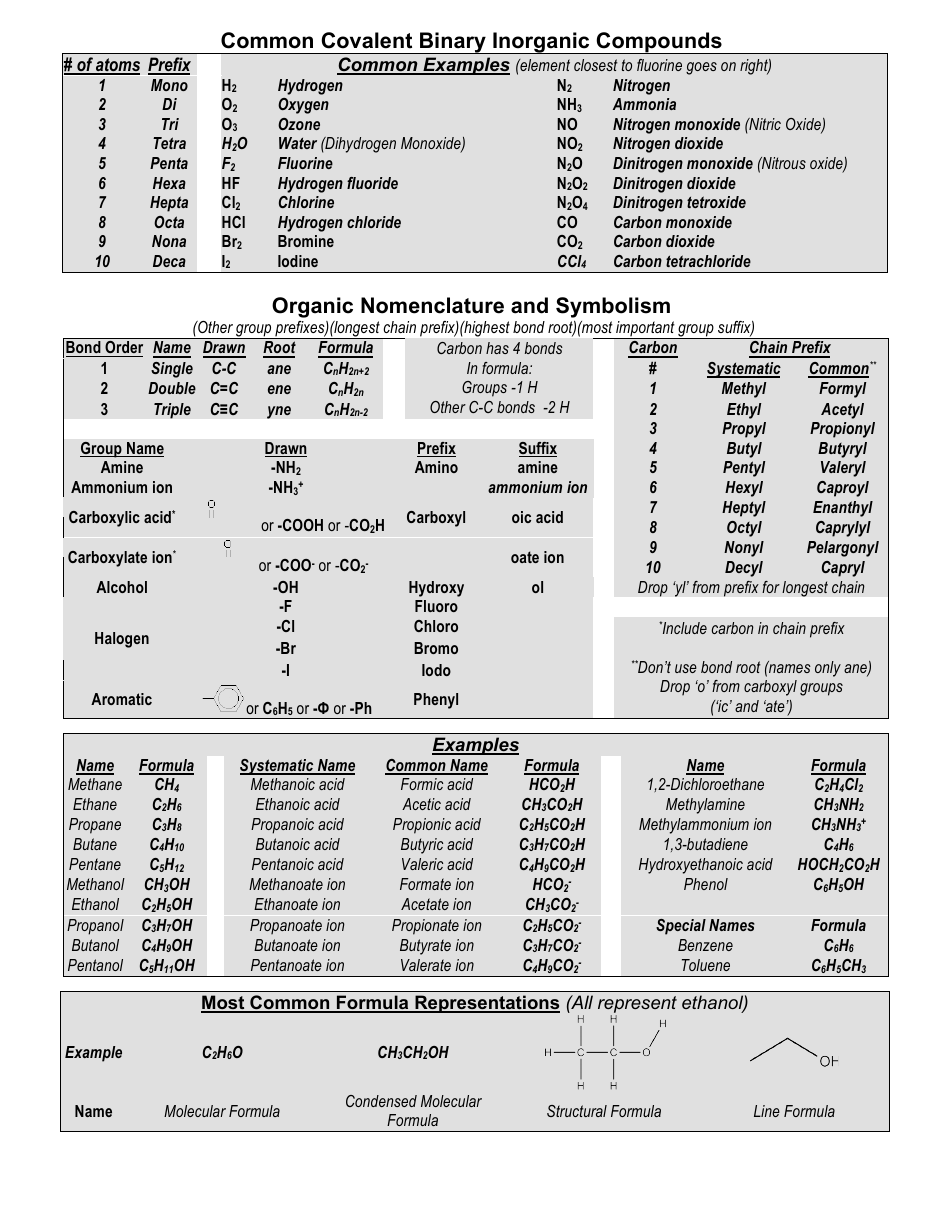
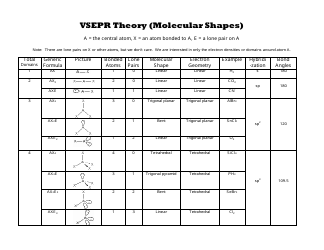
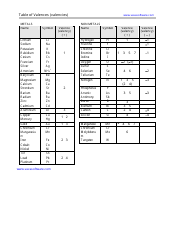
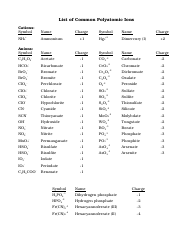
Common Cations, Anions, Acids, Salts and Hydrate Nomenclature Chart
The Common Cations, Anions, Acids, Salts, and Hydrate Nomenclature Chart is used to help identify and name different chemical compounds. It provides a reference for cations (positively charged ions), anions (negatively charged ions), acids, salts, and hydrates, and their corresponding names and formulas.
FAQ
Q: What are cations?
A: Cations are positively charged ions.
Q: What are anions?
A: Anions are negatively charged ions.
Q: What is the nomenclature for acids?
A: The nomenclature for acids typically involves the use of prefixes such as "hydro-" and the suffix "-ic" or "-ous.
Q: What is the nomenclature for salts?
A: The nomenclature for salts usually involves the name of the cation followed by the name of the anion.
Q: What are hydrates?
A: Hydrates are compounds that contain water molecules within their crystal structures.
Q: How are hydrates named?
A: Hydrates are named by adding a prefix to the name of the compound indicating the number of water molecules present.