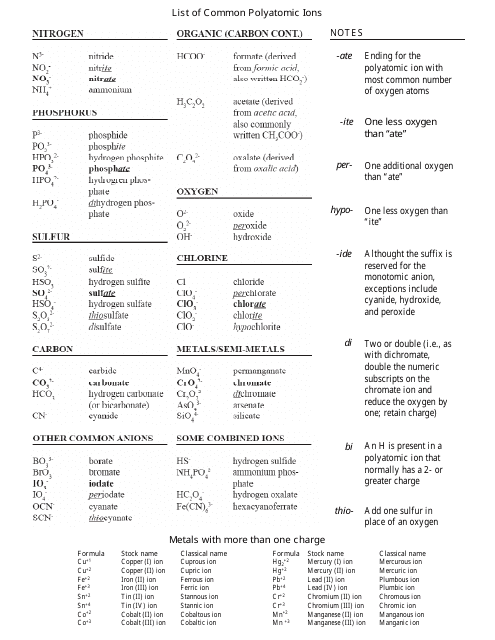
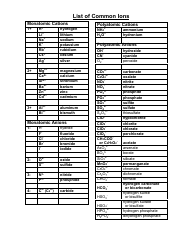
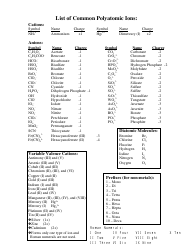
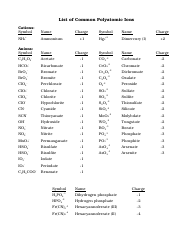
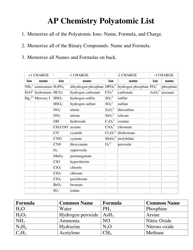
Common Polyatomic Ions List
The Common Polyatomic Ions List is used to identify and classify various charged molecules that are composed of two or more atoms. These ions commonly occur in chemical reactions and play important roles in various fields of science, such as chemistry and biochemistry.
The Common Polyatomic Ions List is not filed by a specific entity or organization. It is a reference list that is widely used in chemistry education and is available in textbooks, online resources, and study materials.
FAQ
Q: What are polyatomic ions?
A: Polyatomic ions are ions composed of two or more atoms.
Q: Why are polyatomic ions important?
A: Polyatomic ions play a crucial role in chemistry, as they are often involved in chemical reactions and the formation of compounds.
Q: What are some examples of common polyatomic ions?
A: Some examples of common polyatomic ions include sulfate (SO4^2-), nitrate (NO3^-), carbonate (CO3^2-), and ammonium (NH4^+) ions.
Q: What is the charge of polyatomic ions?
A: The charge of polyatomic ions can vary, but they are usually negatively charged (anions). However, there are also positively charged (cations) polyatomic ions, such as ammonium (NH4^+).
Q: How are polyatomic ions named?
A: Polyatomic ions are named based on their composition and charge. The names of anions typically end in -ate or -ite, depending on the number of oxygen atoms present.