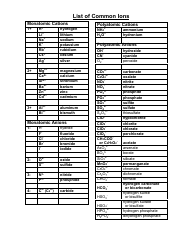
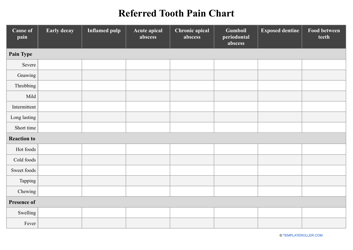
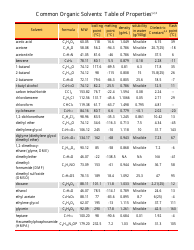
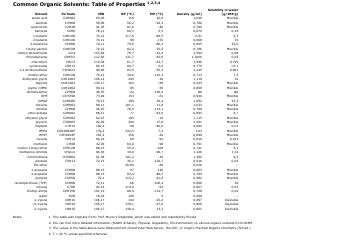
Common Polyatomic Ions Chart - Tables
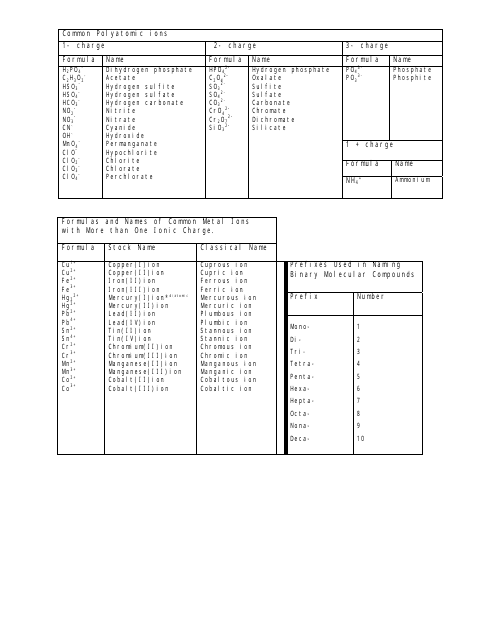
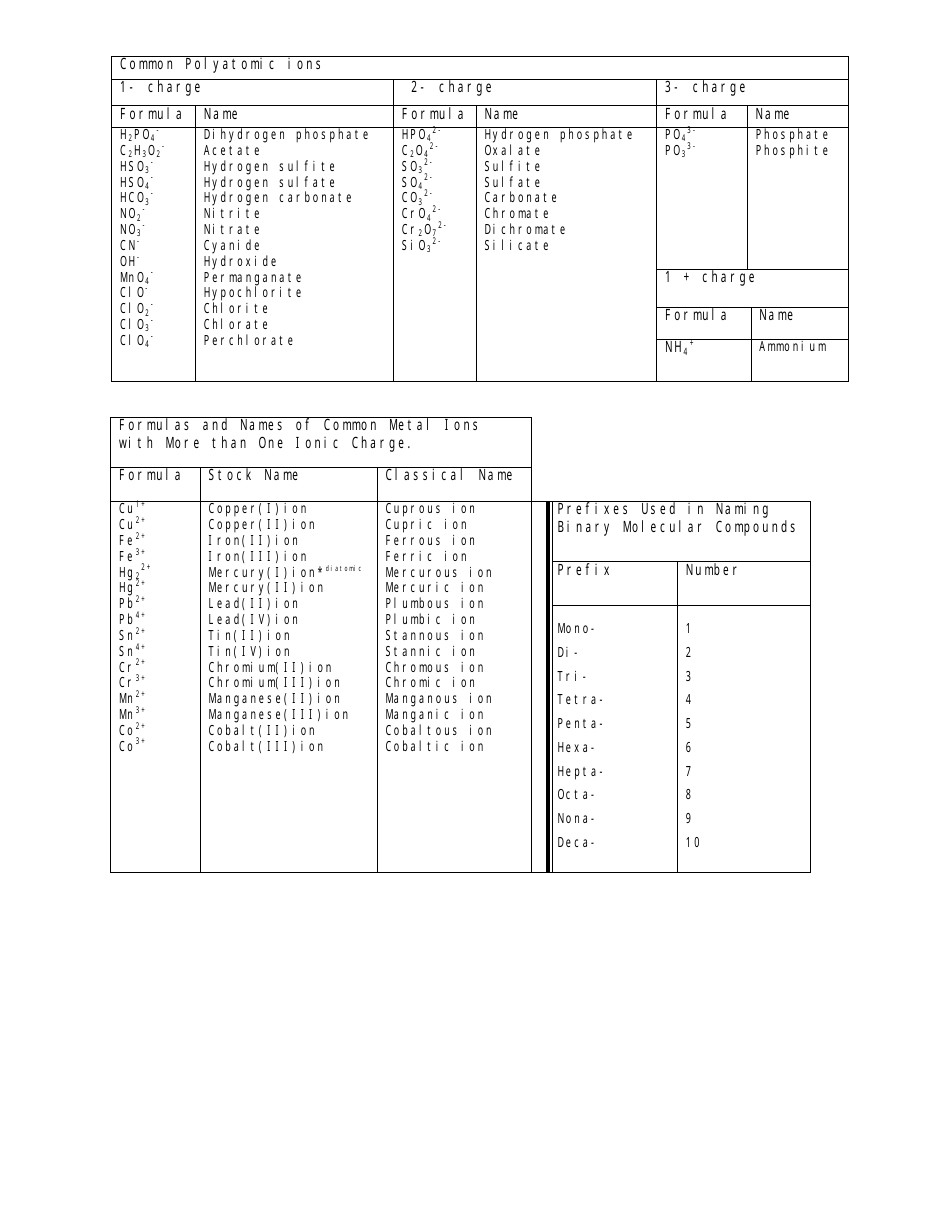
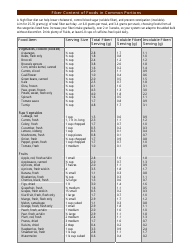
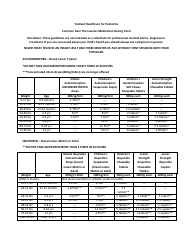
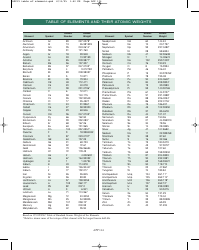
The Common Polyatomic Ions Chart or Tables is used for reference and identification of common polyatomic ions. It provides a list of these ions, along with their chemical formulas and charges. This chart is useful in chemistry for understanding and predicting chemical reactions and balancing equations.
The Common Polyatomic Ions Chart - Tables are not filed by any specific individual or organization. They are widely available and used as a reference in chemistry textbooks, laboratories, and educational resources.
FAQ
Q: What are polyatomic ions?
A: Polyatomic ions are groups of atoms that carry a positive or negative charge.
Q: Can you give some examples of common polyatomic ions?
A: Yes, for example, sulfate (SO4^2-), nitrate (NO3^-), and carbonate (CO3^2-) are common polyatomic ions.
Q: Is it possible for polyatomic ions to have a positive charge?
A: Yes, polyatomic ions can have a positive charge. For example, ammonium (NH4+) is a polyatomic ion with a positive charge.
Q: What is the charge of hydroxide ion?
A: The hydroxide ion (OH-) has a charge of -1.
Q: Are polyatomic ions found in nature?
A: Yes, polyatomic ions are found in nature and are an important part of many compounds.
Q: Do polyatomic ions have specific names?
A: Yes, polyatomic ions have specific names, such as phosphate (PO4^3-) and cyanide (CN^-).
Q: Can you determine the charge of a polyatomic ion from its chemical formula?
A: Yes, the charge of a polyatomic ion can be determined from its chemical formula. The charge is indicated by the superscript on the ion.
Q: Are there any common polyatomic ions with a charge of +2?
A: Yes, for example, the carbonate ion (CO3^2-) and the sulfate ion (SO4^2-) both have a charge of -2.