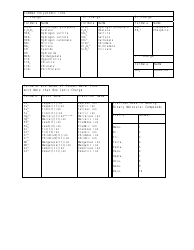
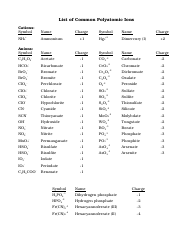
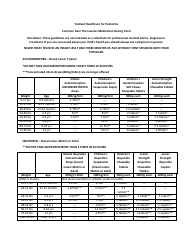
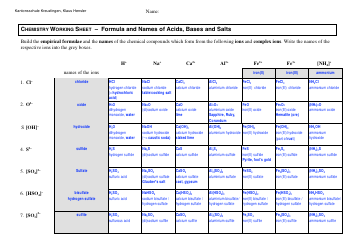
Common Polyatomic Ions Chart - Formulas
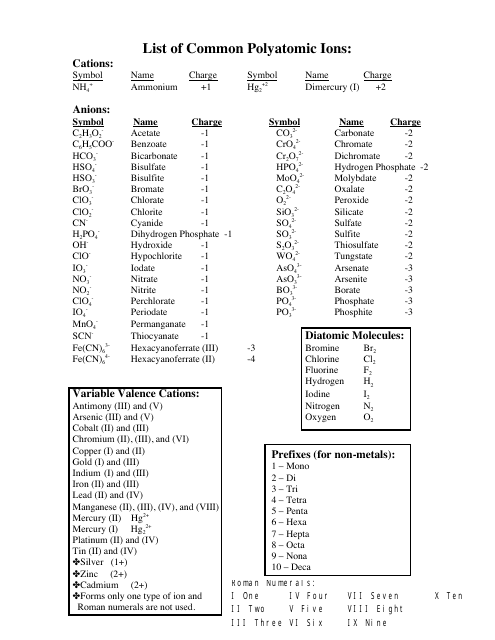
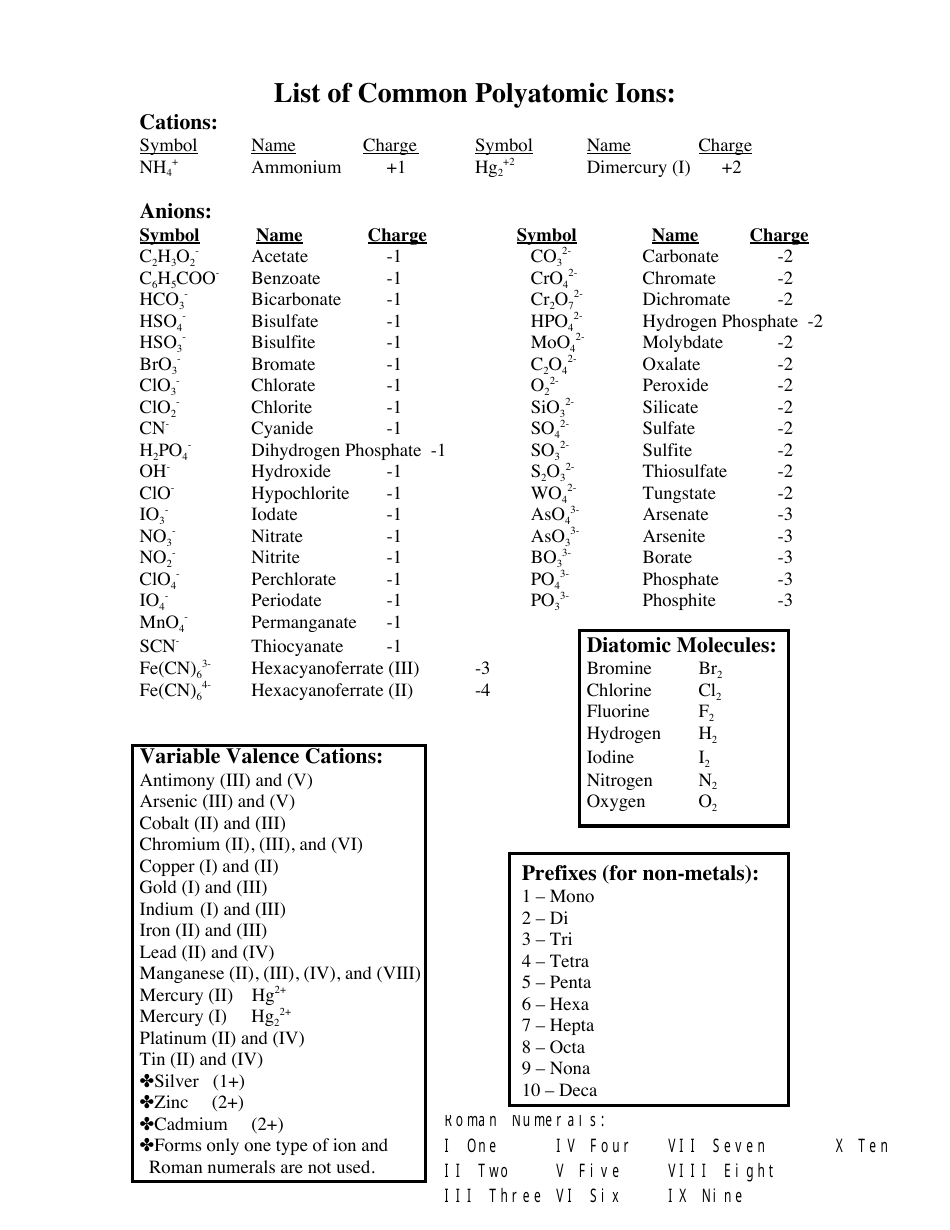
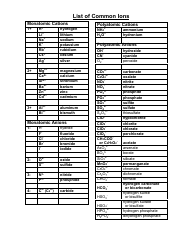
The Common Polyatomic Ions Chart provides a list of common ions with their respective formulas. It is used to help identify and write the formulas for these ions in chemical compounds.
The common polyatomic ions chart - formulas are typically filed and organized by educational institutions, including schools, colleges, and universities.
FAQ
Q: What is a polyatomic ion?
A: A polyatomic ion is a charged particle made up of two or more atoms.
Q: What is the formula for the nitrate ion?
A: The formula for the nitrate ion is NO3^-.
Q: What is the formula for the sulfate ion?
A: The formula for the sulfate ion is SO4^2-.
Q: What is the formula for the phosphate ion?
A: The formula for the phosphate ion is PO4^3-.
Q: What is the formula for the carbonate ion?
A: The formula for the carbonate ion is CO3^2-.
Q: What is the formula for the hydroxide ion?
A: The formula for the hydroxide ion is OH^-.
Q: What is the formula for the ammonium ion?
A: The formula for the ammonium ion is NH4^+.
Q: What is the formula for the cyanide ion?
A: The formula for the cyanide ion is CN^-.
Q: What is the formula for the acetate ion?
A: The formula for the acetate ion is C2H3O2^-.
Q: What is the formula for the perchlorate ion?
A: The formula for the perchlorate ion is ClO4^-.