Percent Ionic Character Worksheet With Answers
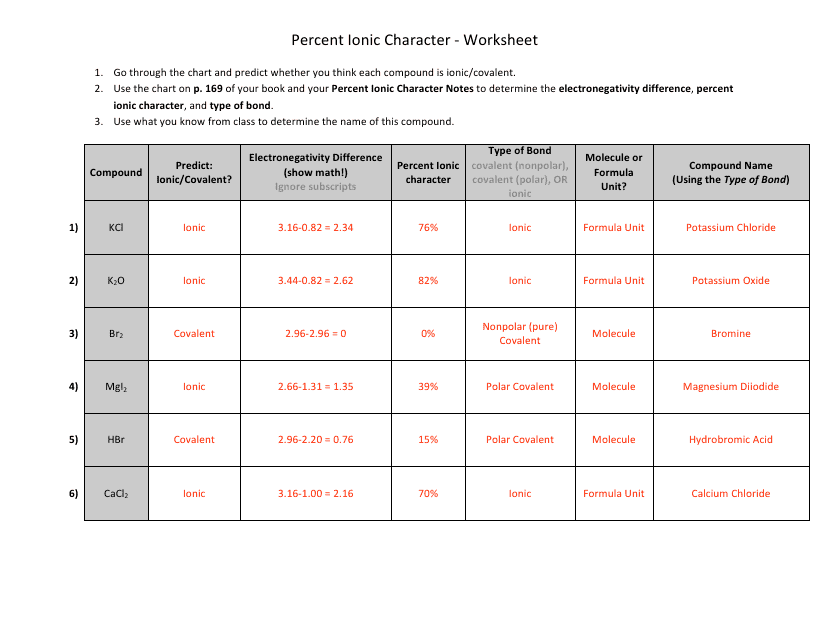
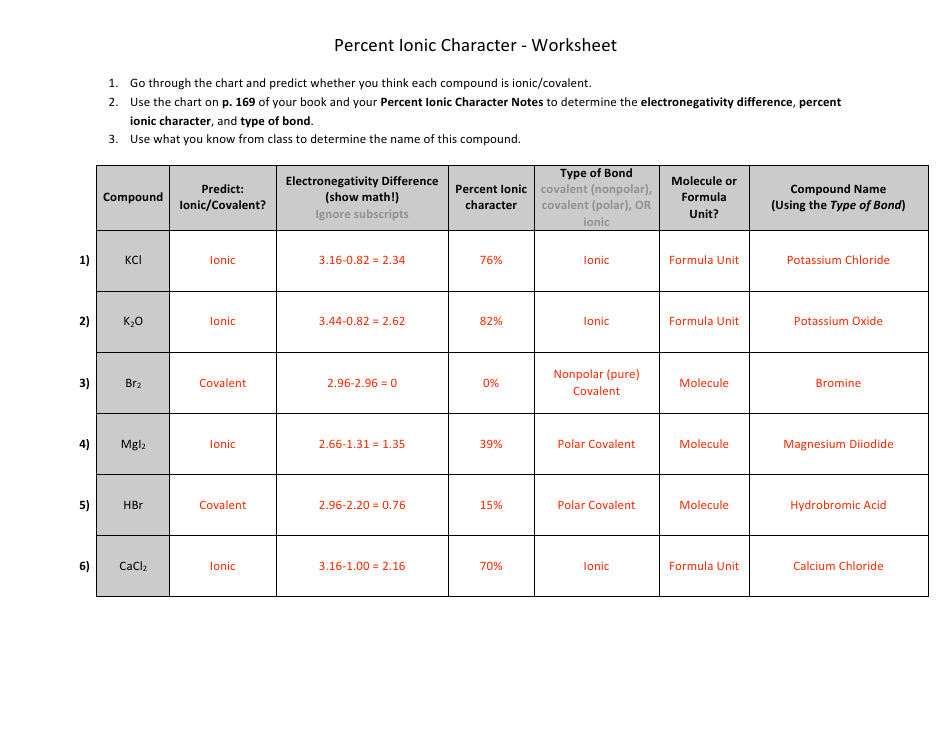
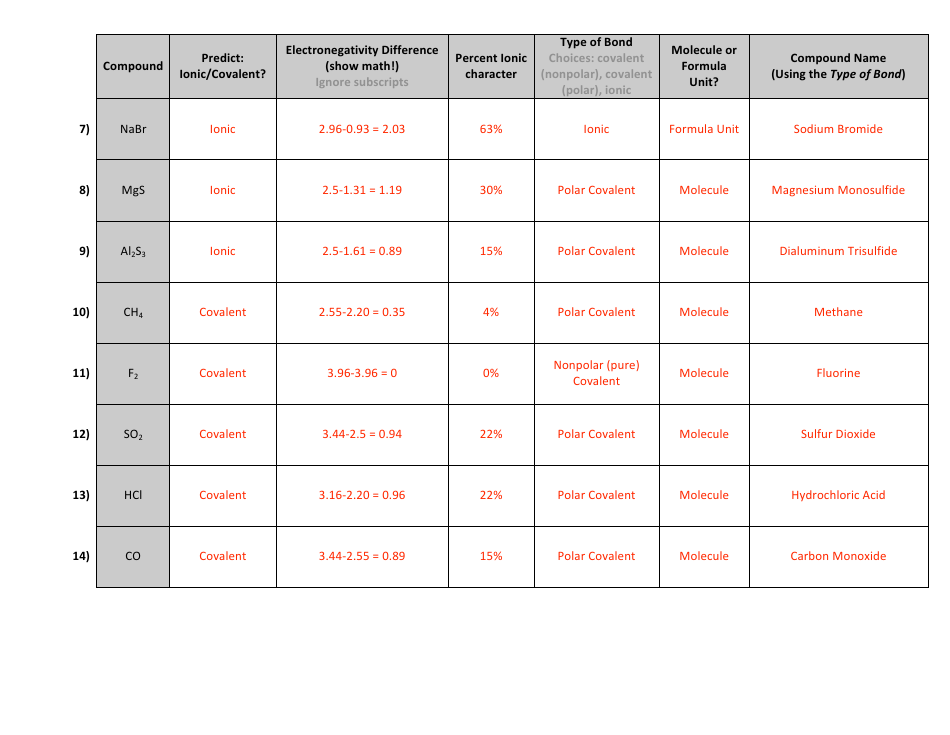
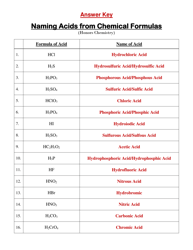
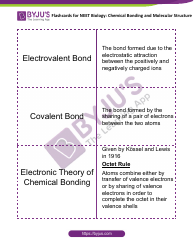
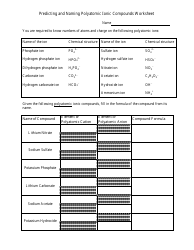
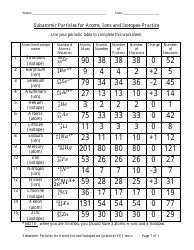
The "Percent Ionic Character Worksheet With Answers" is a document that provides practice problems and solutions related to calculating the percentage of ionic character in a chemical bond. It is likely used in chemistry education to help students understand and apply concepts related to ionic and covalent bonding. This worksheet allows students to practice determining the degree to which a bond is ionic or covalent based on the electronegativity difference between the bonding atoms.
FAQ
Q: What is percent ionic character?
A: Percent ionic character is a measure of the degree of ionic character in a chemical bond between two atoms. It indicates the extent to which electrons are shared or transferred between atoms in a compound.
Q: How is percent ionic character calculated?
A: Percent ionic character can be calculated using the Pauling electronegativity values for the atoms involved in the bond. The formula is:
Q: What does a higher percent ionic character indicate?
A: A higher percent ionic character suggests a bond with greater ionic nature, meaning electrons are more likely to be transferred from one atom to another.
Q: What does a lower percent ionic character indicate?
A: A lower percent ionic character suggests a bond with greater covalent nature, meaning electrons are more likely to be shared between the two atoms.