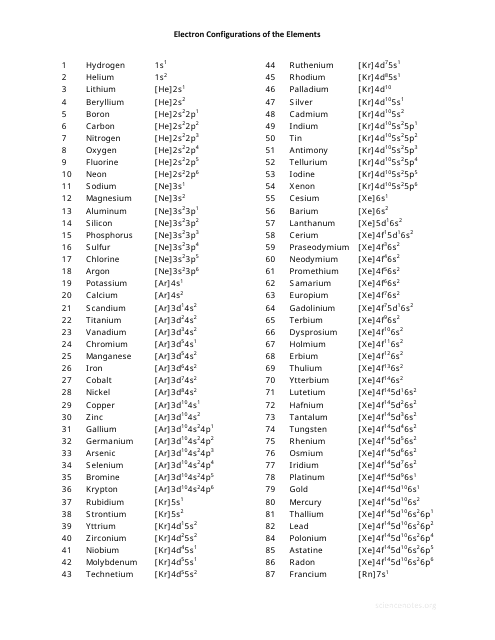
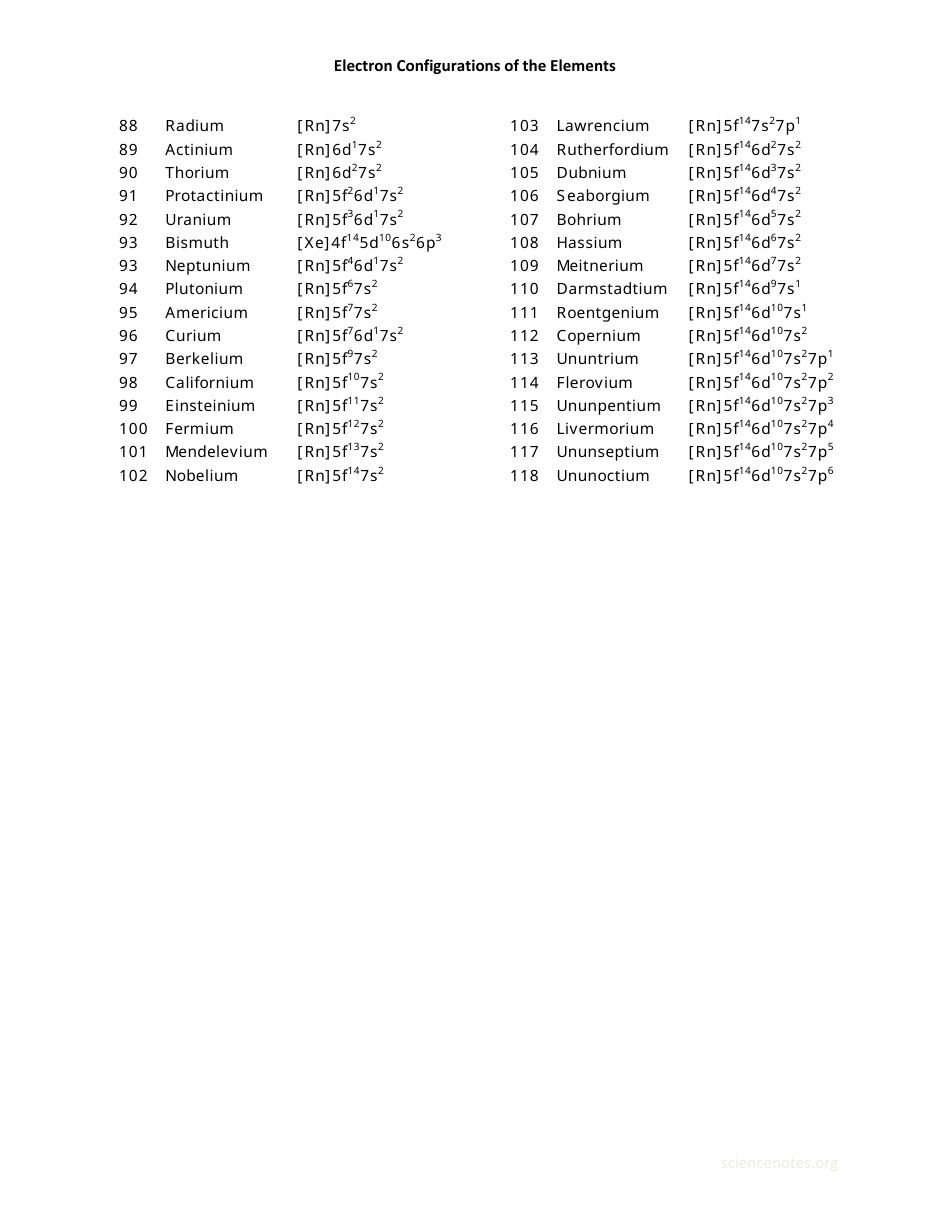
Electron Configurations Chart of the Elements
The Electron Configurations Chart of the Elements is used to display the arrangement of electrons in the energy levels or orbitals of atoms. It helps to understand the organization of the periodic table and the properties of elements.
The electron configurations chart of the elements is typically compiled and maintained by chemists and physicists.
FAQ
Q: What is an electron configuration?
A: An electron configuration describes how electrons are arranged in an atom.
Q: Why is electron configuration important?
A: Electron configuration is important because it determines an element's chemical properties and behavior.
Q: What does the electron configuration chart show?
A: The electron configuration chart shows the arrangement of electrons in the orbitals of each element.
Q: How are electron configurations written?
A: Electron configurations are written using a series of numbers and letters that represent the energy levels and sublevels.
Q: What are the main types of orbitals?
A: The main types of orbitals are s, p, d, and f orbitals.
Q: What is the Aufbau principle?
A: The Aufbau principle states that electrons fill orbitals in order of increasing energy.
Q: What is Hund's rule?
A: Hund's rule states that electrons occupy orbitals of the same energy level singly before pairing up.
Q: What is the Pauli exclusion principle?
A: The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers.