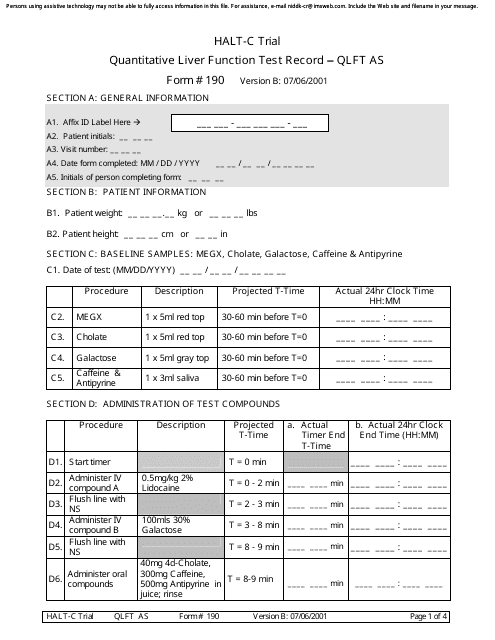
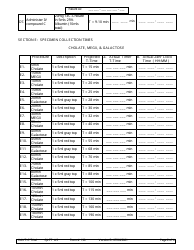
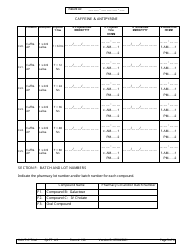
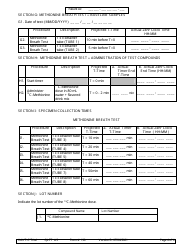
Form 190 Quantitative Liver Function Test Record
What Is Form 190?
This is a legal form that was released by the U.S. Department of Health and Human Services on July 6, 2001 and used country-wide. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is Form 190?
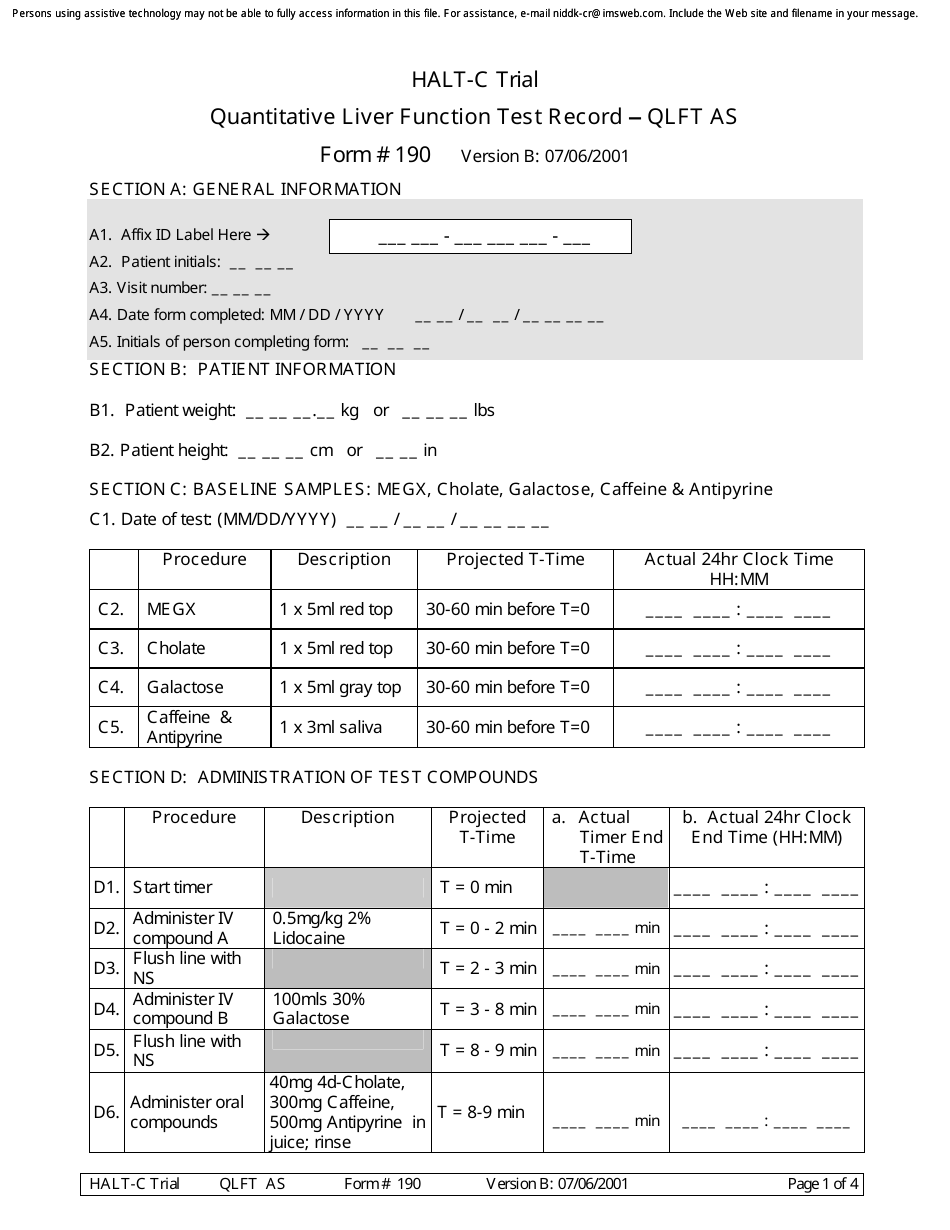
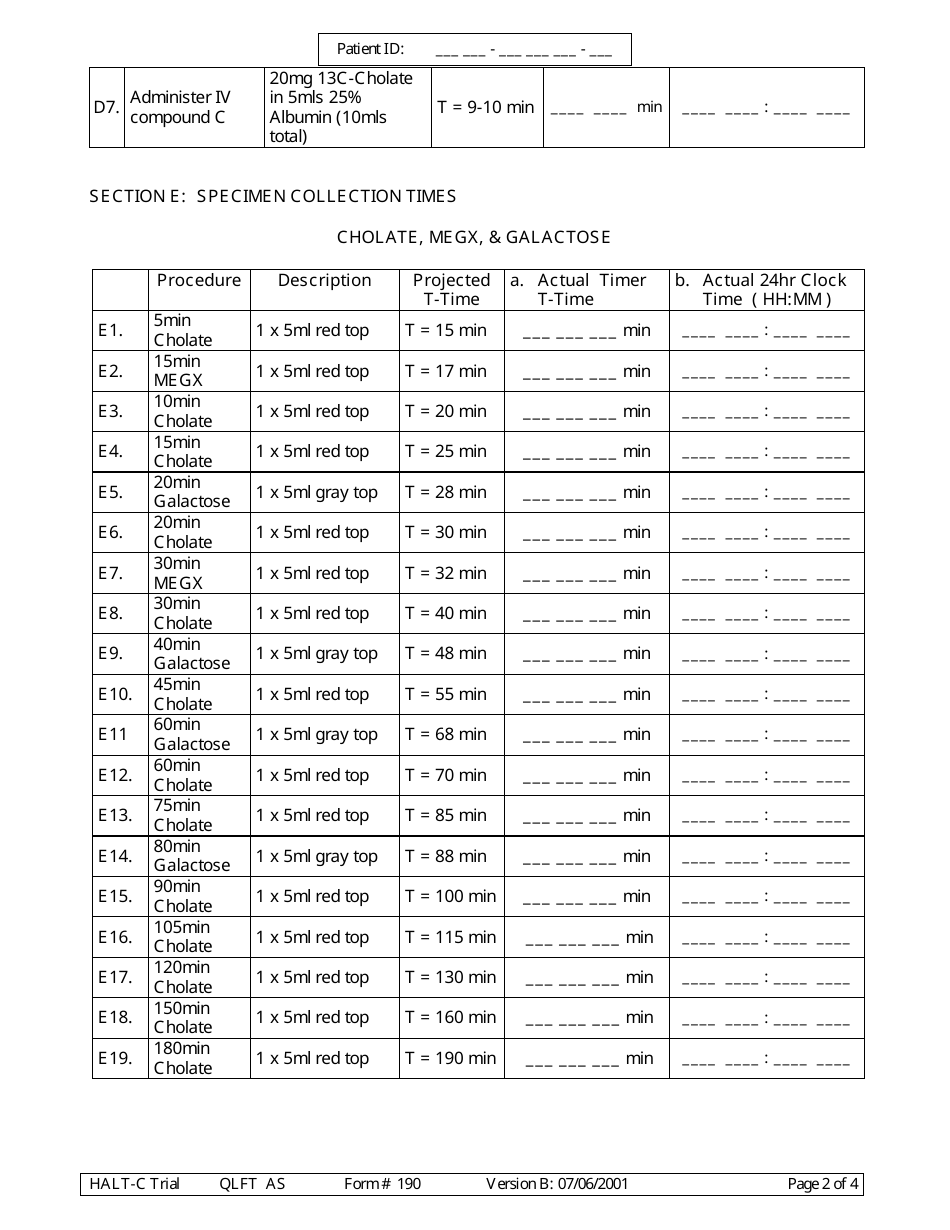
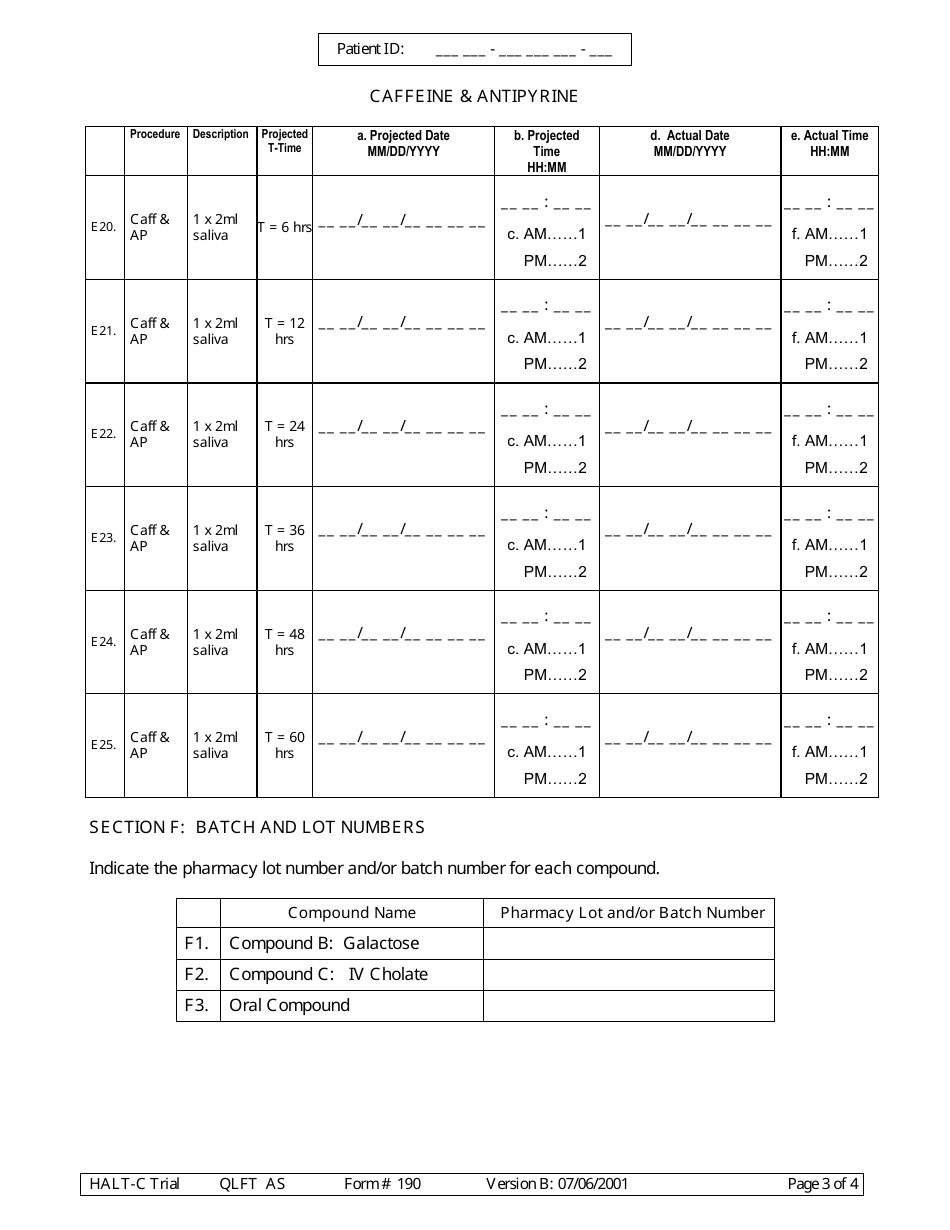
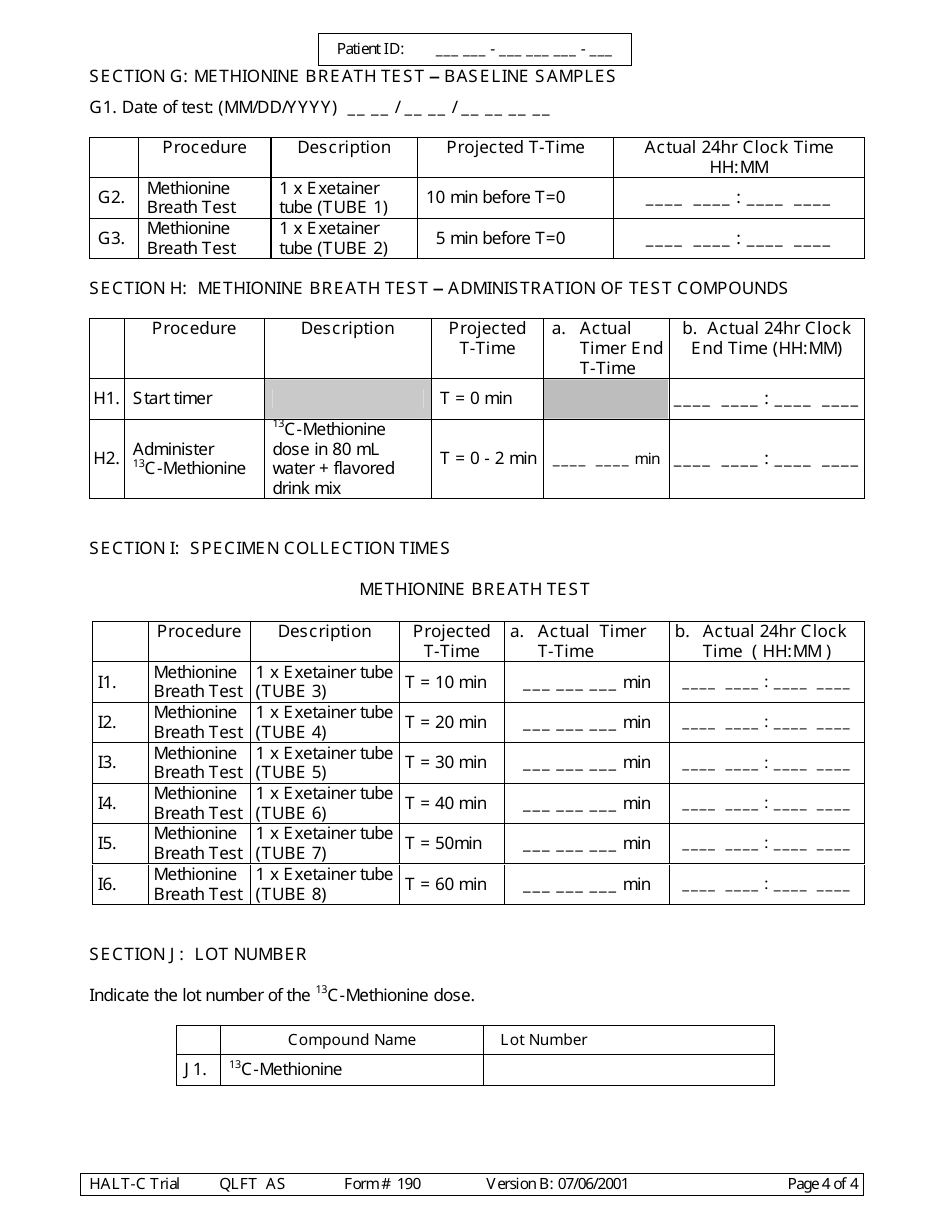
A: Form 190 is a document used to record quantitative liver function test results.
Q: What are quantitative liver function tests?
A: Quantitative liver function tests are laboratory tests that measure specific substances in the blood to assess the overall function of the liver.
Q: Why are quantitative liver function tests done?
A: Quantitative liver function tests are done to evaluate the health and function of the liver, diagnose liver diseases, monitor treatment effectiveness, and assess liver damage.
Q: What information is recorded on Form 190?
A: Form 190 records the results of quantitative liver function tests, including measurements of bilirubin, albumin, alanine transaminase (ALT), and aspartate transaminase (AST).
Q: How often are quantitative liver function tests performed?
A: The frequency of quantitative liver function tests depends on the individual's medical condition and the recommendations of their healthcare provider.
Q: Do quantitative liver function tests require any special preparation?
A: Some quantitative liver function tests may require fasting or specific medication instructions. It is best to follow the instructions provided by the healthcare provider.
Q: Can the results of quantitative liver function tests diagnose a specific liver disease?
A: While abnormal results of quantitative liver function tests can indicate liver dysfunction, they are not specific to a particular liver disease. Additional tests may be needed to make a definitive diagnosis.
Form Details:
- Released on July 6, 2001;
- The latest available edition released by the U.S. Department of Health and Human Services;
- Easy to use and ready to print;
- Yours to fill out and keep for your records;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a printable version of Form 190 by clicking the link below or browse more documents and templates provided by the U.S. Department of Health and Human Services.