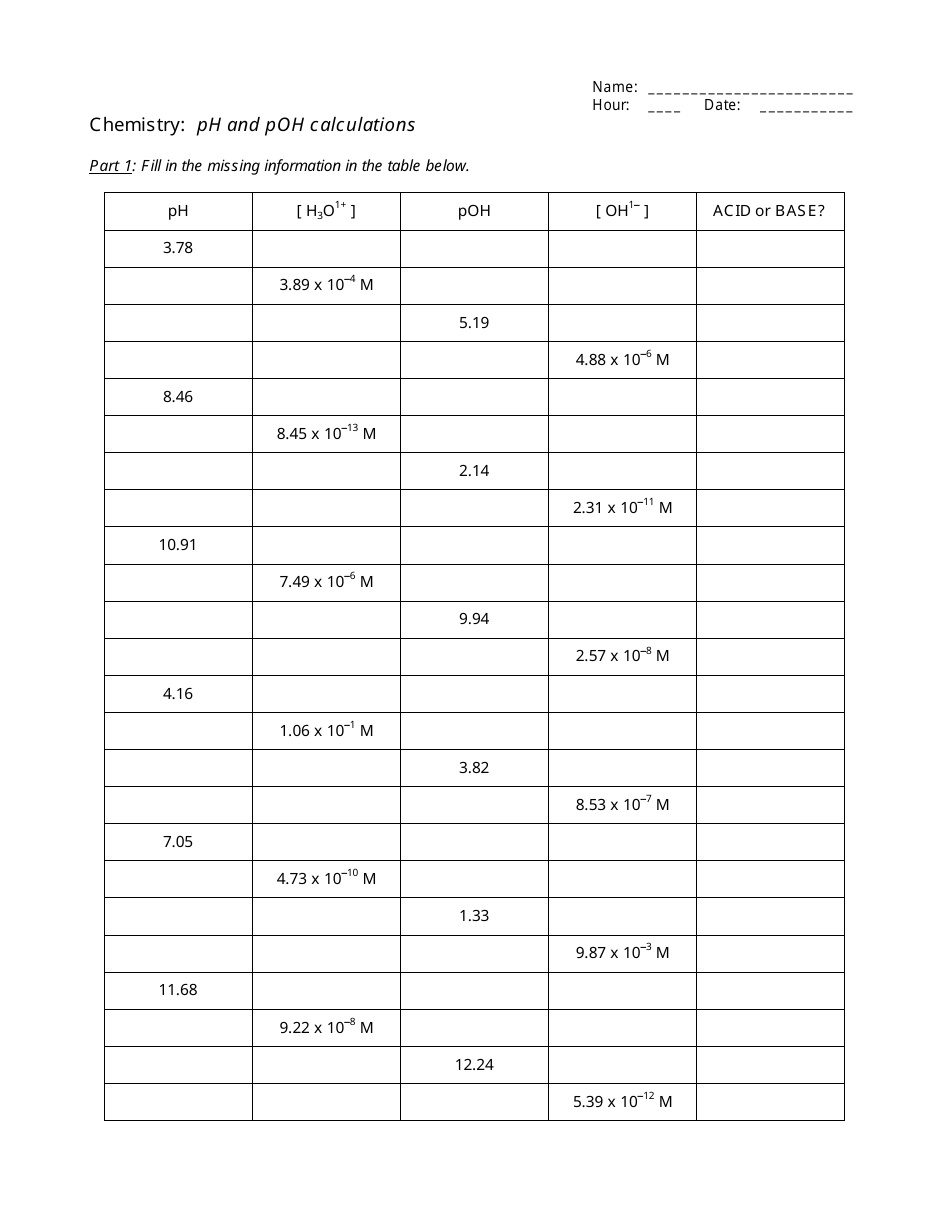
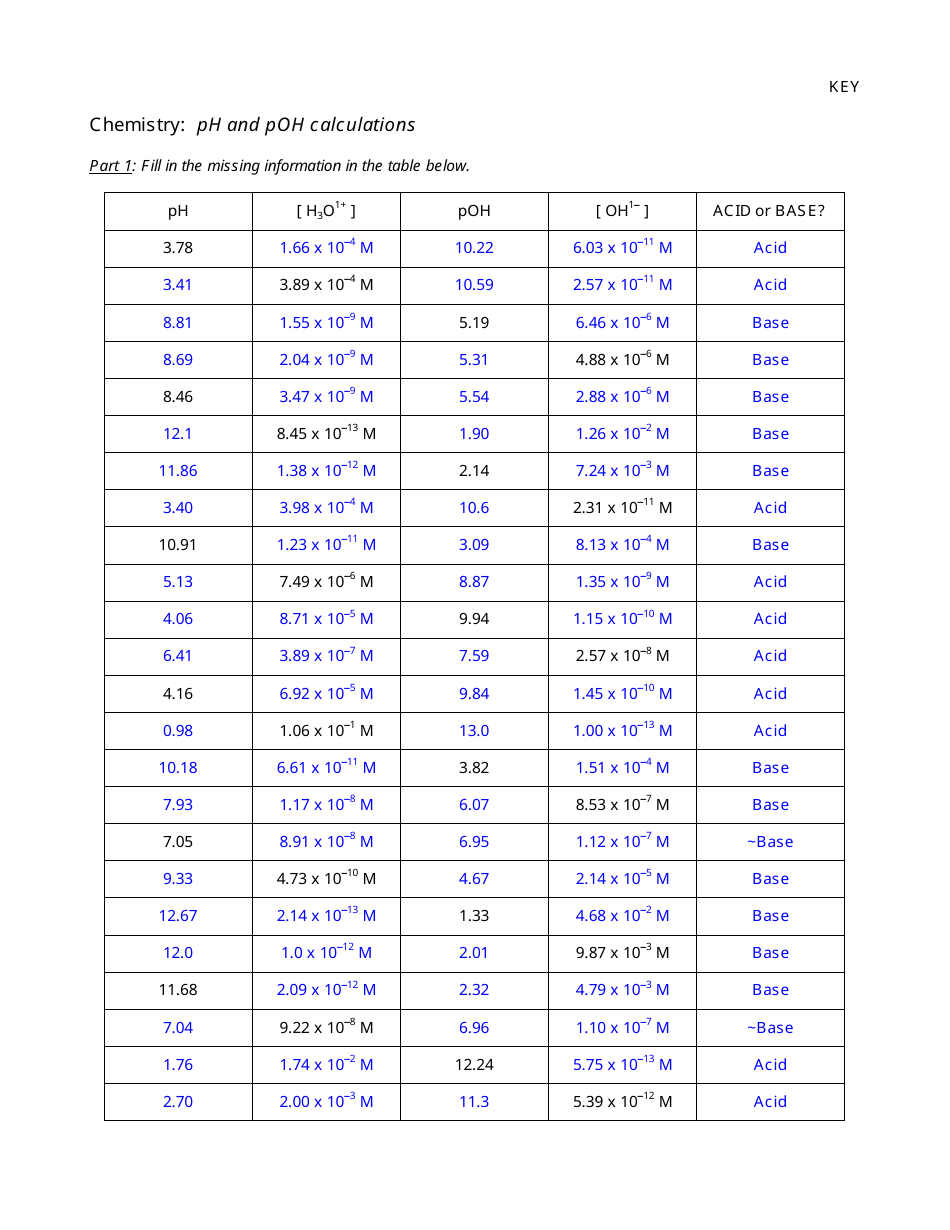
Ph and Poh Calculations Chemistry Worksheet With Answers
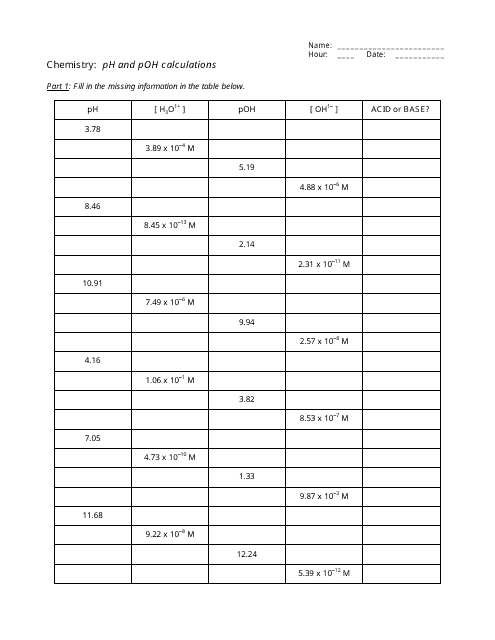
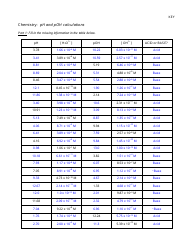
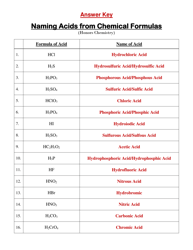
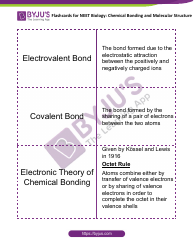
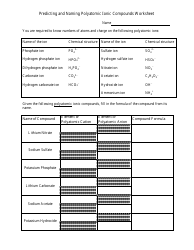
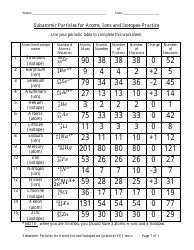
The "Ph and Poh Calculations Chemistry Worksheet With Answers" is a resource used to practice calculating the pH and pOH values in chemistry. It helps students understand the acidity and alkalinity of solutions.
The pH and pOH calculations chemistry worksheet with answers would typically be filed by the student or in some cases, by the teacher or school administration.
FAQ
Q: What is pH?
A: pH is a measure of the acidity or alkalinity of a solution.
Q: How is pH calculated?
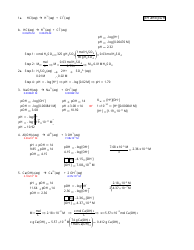
A: pH is calculated using the formula: pH = -log[H+], where [H+] represents the concentration of hydrogen ions in the solution.
Q: What is pOH?
A: pOH is a measure of the concentration of hydroxide ions in a solution.
Q: How is pOH calculated?
A: pOH is calculated using the formula: pOH = -log[OH-], where [OH-] represents the concentration of hydroxide ions in the solution.
Q: What is the relationship between pH and pOH?
A: The sum of pH and pOH for a given solution is always equal to 14.
Q: How can you convert between pH and pOH?
A: To convert between pH and pOH, subtract the given value from 14. For example, if pH is 3, pOH would be 11.
Q: What is a neutral solution?
A: A neutral solution has a pH of 7 and a pOH of 7.
Q: What is an acidic solution?
A: An acidic solution has a pH less than 7 and a pOH greater than 7.
Q: What is a basic (alkaline) solution?
A: A basic solution has a pH greater than 7 and a pOH less than 7.