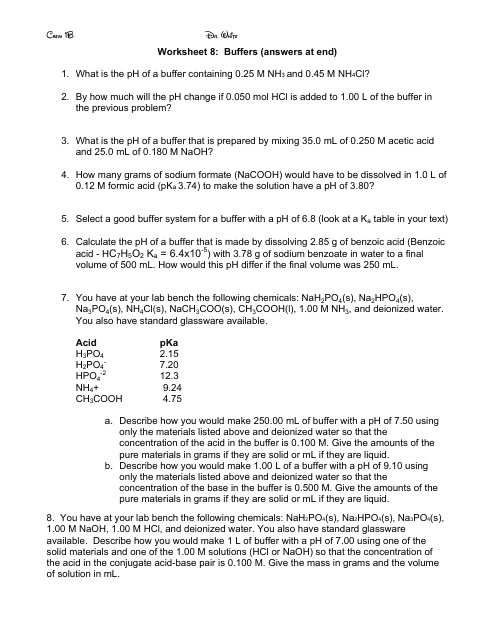
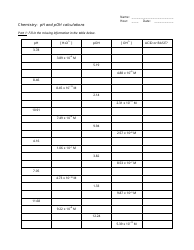
Buffers Chemistry Worksheet With Answers - Dr. White
FAQ
Q: What is a buffer?
A: A buffer is a solution that resists changes in pH when small amounts of acid or base are added.
Q: Why are buffers important?
A: Buffers are important because they help maintain the pH of a solution within a specific range, which is crucial for many biological and chemical processes.
Q: How do buffers work?
A: Buffers work by containing a weak acid and its conjugate base (or a weak base and its conjugate acid). These components can react with added acid or base, preventing large changes in pH.
Q: What is the equation for the Henderson-Hasselbalch equation?
A: The Henderson-Hasselbalch equation is pH = pKa + log([A-]/[HA]), where pH is the desired pH, pKa is the dissociation constant of the weak acid, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the weak acid.
Q: How can buffers be prepared?
A: Buffers can be prepared by mixing a weak acid and its conjugate base, or a weak base and its conjugate acid, in specific ratios to achieve the desired pH.
Q: What is the pH range for most biological buffers?
A: The pH range for most biological buffers is between 6 and 8.
Q: What are some examples of common biological buffers?
A: Some examples of common biological buffers include phosphate buffer, Tris buffer, and HEPES buffer.
Q: Can buffers be used in medical and scientific experiments?
A: Yes, buffers are commonly used in medical and scientific experiments to maintain the pH of solutions and ensure accurate results.
Q: What happens if a buffer is overwhelmed?
A: If a buffer is overwhelmed, the pH of the solution may change significantly, leading to potential disruptions in biological or chemical processes.
Q: Are buffers only used in aqueous solutions?
A: Buffers are primarily used in aqueous solutions, as they rely on the ionization of acids and bases in water. However, some non-aqueous buffers exist for specific applications.