Activity Series of Metals and Non-metals Cheat Sheet
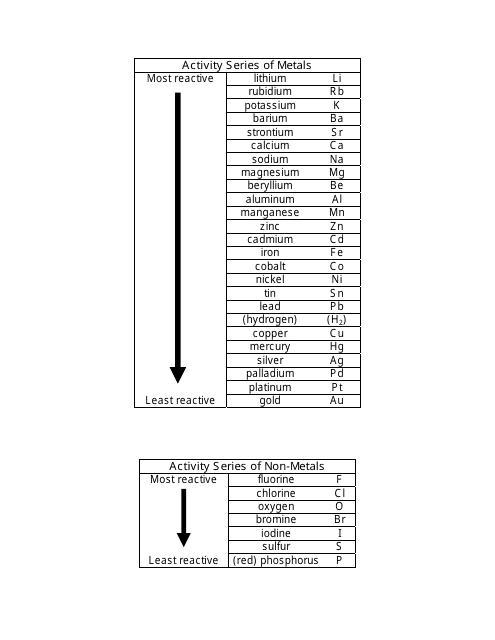
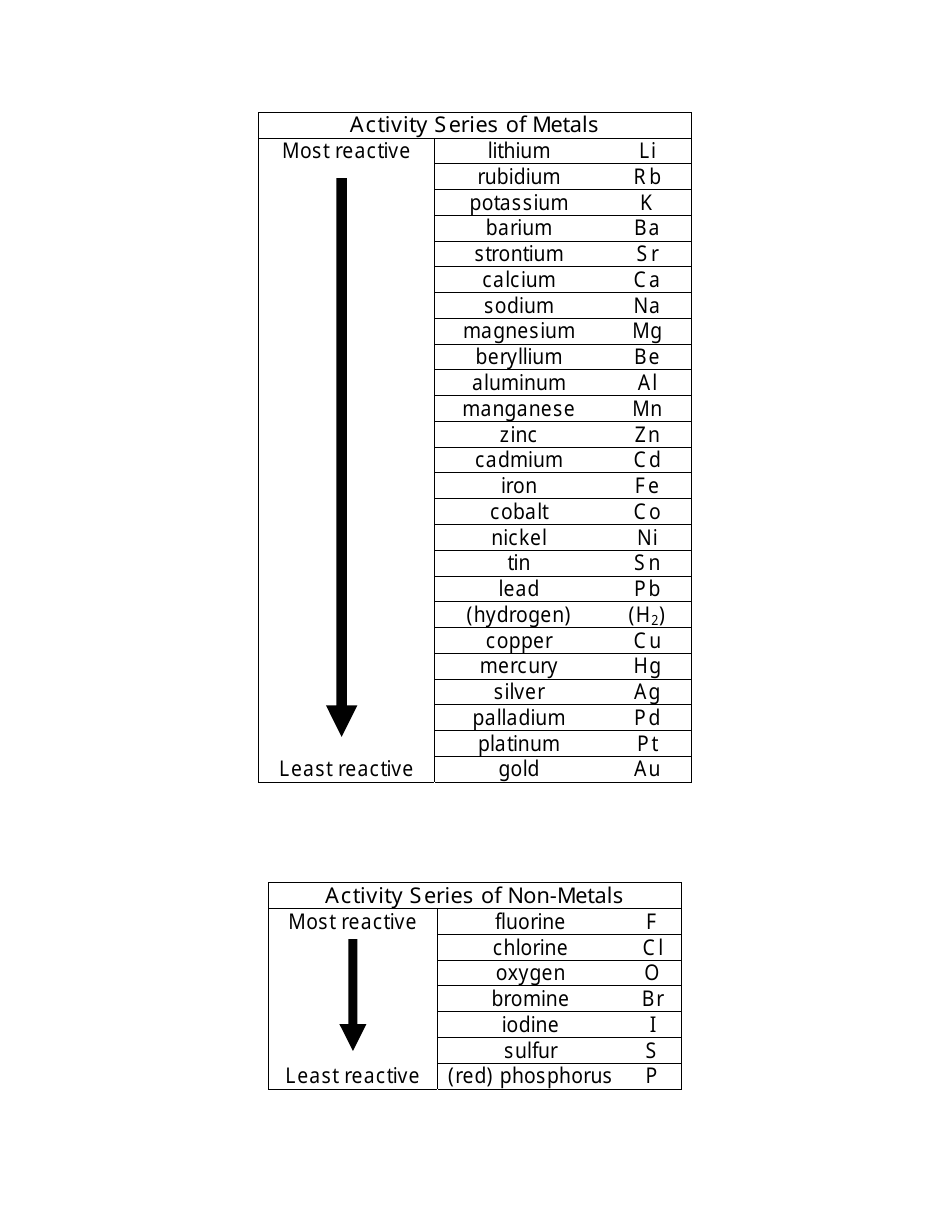
The Activity Series of Metals and Non-metals Cheat Sheet is a quick reference guide that lists the reactivity of metals and non-metals. It helps in predicting the reactions between different elements and their ability to displace each other in chemical reactions.
The activity series of metals and non-metals cheat sheet is not filed by a specific individual or organization. It is a compilation of information based on scientific research and knowledge in the field of chemistry.
FAQ
Q: What is an activity series of metals?
A: An activity series of metals is a list of metals arranged in order of their reactivity.
Q: What is an activity series of non-metals?
A: An activity series of non-metals is a list of non-metals arranged in order of their reactivity.
Q: Why is the activity series important?
A: The activity series is important because it helps predict the outcome of chemical reactions, such as displacement reactions.
Q: What does it mean for a metal to be more reactive?
A: A more reactive metal will displace a less reactive metal from a compound during a displacement reaction.
Q: What does it mean for a non-metal to be more reactive?
A: A more reactive non-metal will displace a less reactive non-metal from a compound during a displacement reaction.
Q: How can the activity series be used?
A: The activity series can be used to determine the feasibility and outcome of chemical reactions, as well as to predict which metals can displace other metals from compounds.
Q: What are some examples of metals in the activity series?
A: Examples of metals in the activity series include potassium, sodium, magnesium, aluminum, zinc, iron, copper, silver, and gold.
Q: What are some examples of non-metals in the activity series?
A: Examples of non-metals in the activity series include fluorine, chlorine, bromine, iodine, and oxygen.
Q: Why are noble gases not included in the activity series?
A: Noble gases, such as helium and neon, are not included in the activity series because they are extremely stable and unreactive.
Q: What happens when a metal reacts with an acid?
A: When a metal reacts with an acid, it displaces hydrogen gas and forms a salt.
Q: What happens when a non-metal reacts with an acid?
A: Non-metals do not react with acids in the same way as metals. Instead, they may undergo other types of reactions such as oxidation or reduction.