Counting Atoms in Compounds Chemistry Worksheet - West Linn-Wilsonville School District
The Counting Atoms in Compounds Chemistry Worksheet is a learning resource used by the West Linn-Wilsonville School District to teach students about counting the number of atoms in different compounds in the subject of Chemistry.
FAQ
Q: What is the purpose of the Counting Atoms in Compounds Chemistry Worksheet?
A: The purpose of the worksheet is to practice counting atoms in compounds.
Q: What is a compound in chemistry?
A: A compound is a substance made up of two or more different elements chemically bonded together.
Q: Why is it important to be able to count atoms in compounds?
A: Counting atoms helps in understanding the composition and properties of the compound.
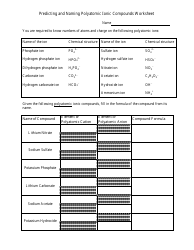
Q: How is the number of atoms in a compound determined?
A: The number of atoms in a compound is determined by the chemical formula and the number of each element present.
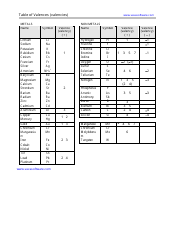
Q: What is a chemical formula?
A: A chemical formula is a representation of the elements present in a compound and their respective ratios.
Q: What is the difference between a subscript and a coefficient in a chemical formula?
A: A subscript represents the number of atoms of an element in a compound, while a coefficient represents the number of molecules.
Q: How are atoms counted in compounds?
A: Atoms are counted by multiplying the subscript of each element by the coefficient, if present, in the chemical formula.
Q: Can the order of elements in a chemical formula be changed?
A: No, the order of elements in a chemical formula is fixed and represents the specific arrangement of atoms in the compound.
Q: What are some common examples of compounds?
A: Water (H2O), carbon dioxide (CO2), and sodium chloride (NaCl) are common examples of compounds.
Q: What is the significance of understanding the composition of compounds?
A: Understanding the composition of compounds is crucial for various applications, such as manufacturing, medicine, and environmental studies.
![[School Name] Counting Atoms in Compounds Chemistry Worksheet Preview - West Linn-Wilsonville School District](https://data.templateroller.com/pdf_docs_html/300/3000/300006/counting-atoms-in-compounds-chemistry-worksheet-west-linn-wilsonville-school-district_big.png)


![[School Name] Counting Atoms in Compounds Chemistry Worksheet Preview - West Linn-Wilsonville School District](https://data.templateroller.com/pdf_docs_html/300/3000/300006/page_1_thumb_950.png)