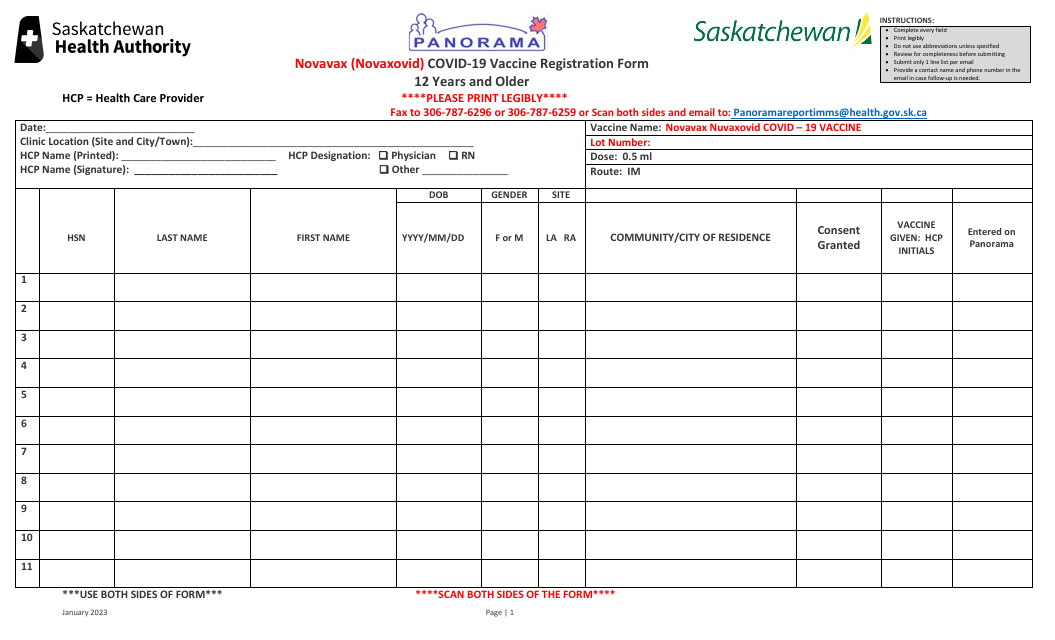
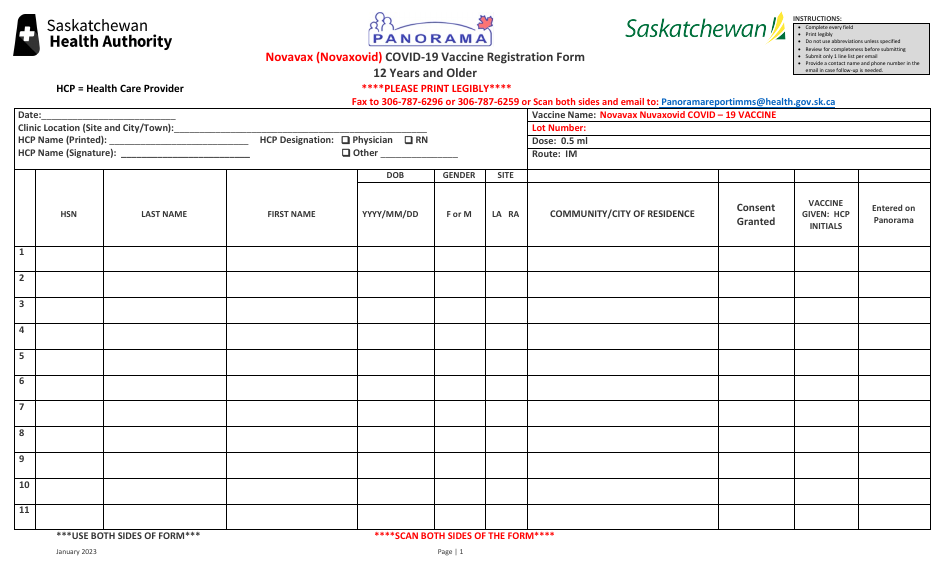
Novavax (Novaxovid) Covid-19 Vaccine Registration Form - 12 Years and Older - Saskatchewan, Canada
The Novavax (Novaxovid) Covid-19 Vaccine Registration Form - 12 Years and Older - Saskatchewan, Canada is a document that residents in Saskatchewan, Canada need to fill out to register for the Novavax (Novaxovid) Covid-19 vaccine. This specific form applies to individuals who are at least 12 years old. The form includes various sections such as personal identification information, medical history, and consent to get the Novavax vaccine. The purpose of this form is to record necessary health details, ensure the candidate's eligibility for the vaccine, and to schedule their vaccination appointment.
The Novavax (Nuvaxovid) Covid-19 Vaccine Registration Form - 12 Years and Older - Saskatchewan, Canada should be filed by the individual receiving the vaccine if they are 18 years old or above.
If the individual is between 12 and 17 years old, the form should be filled out and signed by one of their parents or a legal guardian. Some provinces also require the consent of the minor if they are capable of providing it, depending on local laws.
Please note that it's important to check with local health authorities or a healthcare provider for any specific requirements, as policies can vary.
FAQ
Q: What is Novavax (Novaxovid) Covid-19 Vaccine?
A: Novavax (Novaxovid) is a Covid-19 vaccine that has been approved for emergency use in several countries. It is a protein-based vaccine that triggers an immune response in the body to fight against the virus.
Q: Is the Novavax vaccine suitable for children?
A: The registration form specifies that the Novavax (Novaxovid) Covid-19 vaccine is for individuals aged 12 years and older. Therefore, children under 12 should not be given this vaccine.
Q: What is the efficacy of the Novavax (Novaxovid) Covid-19 Vaccine?
A: Novavax's Phase 3 clinical trials have shown that the vaccine is about 90% effective at preventing symptomatic Covid-19 infection.
Q: Is the Novavax (Novaxovid) Covid-19 Vaccine available in the USA, India, and Australia?
A: The availability of the Novavax Covid-19 vaccine varies by country. Each country's health authority makes decisions about vaccine approvals and distributions based on their situations and vaccine supply. In the USA, approval and distribution of Novavax is still pending as of the time of this writing. In India, Novavax has been approved under the name Covovax; and in Australia, the Therapeutic Goods Administration has provisionally approved it for individuals 18 years and older under the brand name Nuvaxovid. However, these situations can change quickly, so one should always check with the appropriate health authorities for the most current information.
Q: What are the potential side effects of the Novavax Covid-19 vaccine?
A: Potential side effects of the Novavax Covid-19 vaccine are similar to other Covid-19 vaccines and may include pain and swelling at the injection site, fatigue, headache, muscle pain, chills, fever, and nausea. These side effects are usually mild to moderate and go away on their own within a few days.