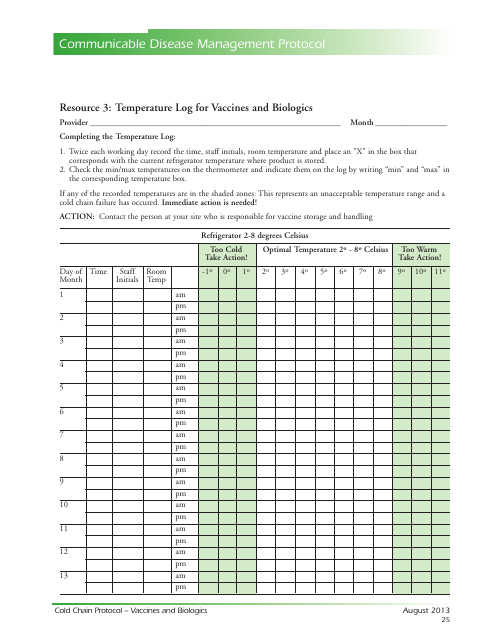
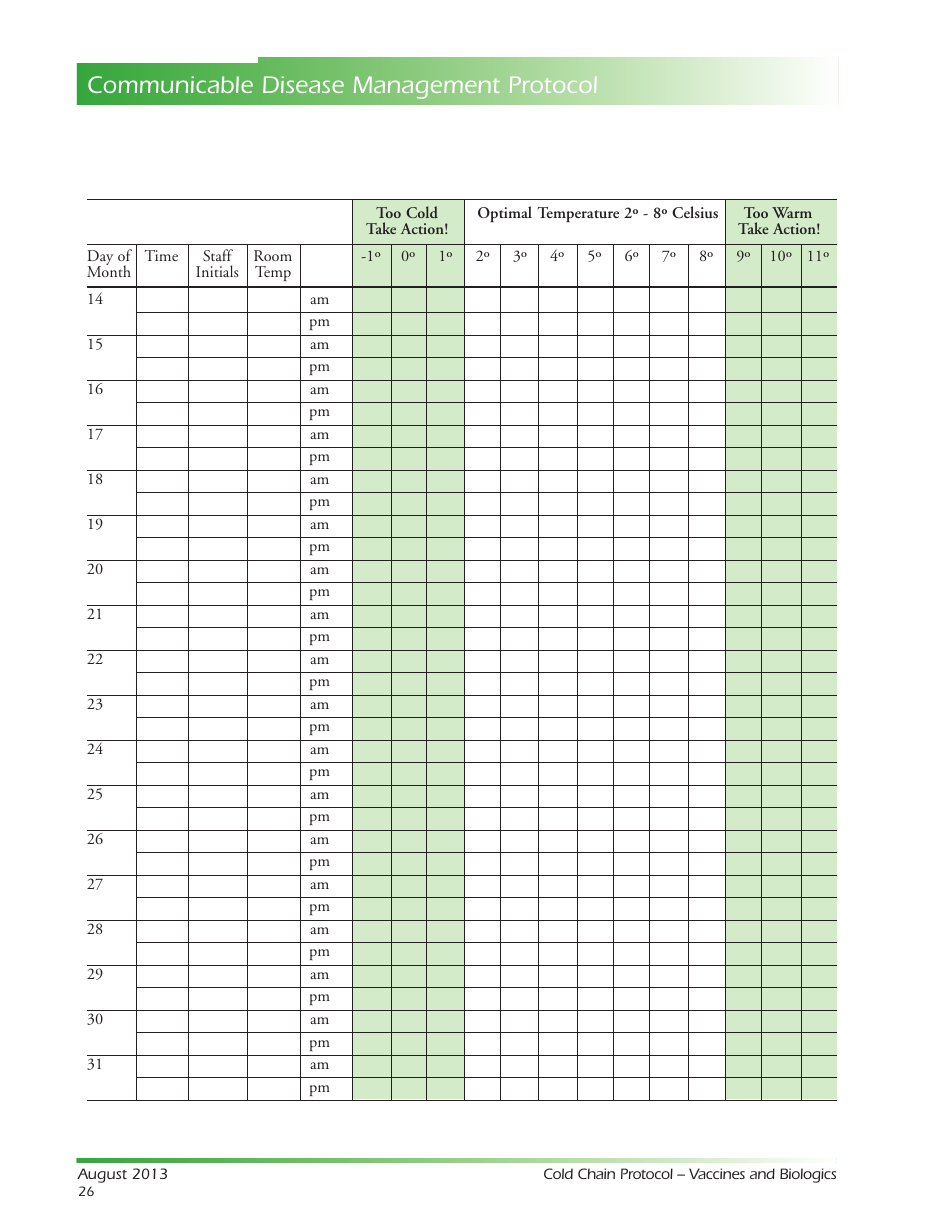
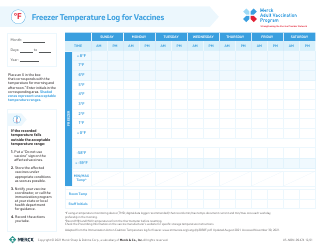
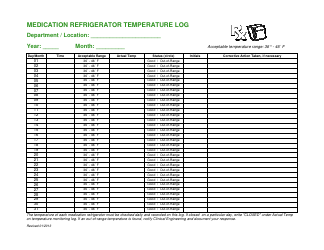
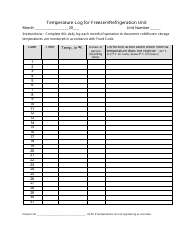
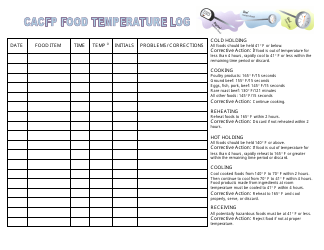
Temperature Log for Vaccines and Biologics
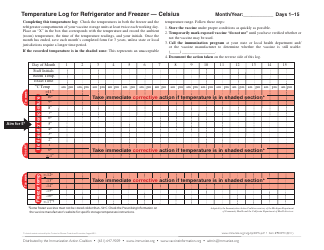
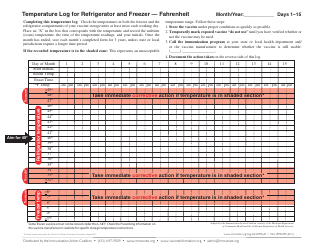
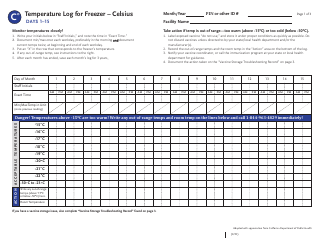
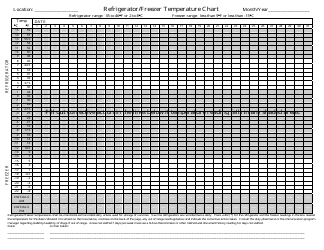
Temperature Log for Vaccines and Biologics is used to ensure that these products are stored and transported under appropriate temperature conditions. It helps monitor and maintain the required temperature range, preserving the effectiveness and safety of vaccines and biologics.
The temperature log for vaccines and biologics is typically filed by the healthcare provider or the facility responsible for storing and administering these products.
FAQ
Q: What is a temperature log?
A: A temperature log is a record that documents the temperature conditions of vaccines and biologics.
Q: Why is a temperature log important?
A: A temperature log is important because it helps ensure that vaccines and biologics are stored and transported within the recommended temperature range to maintain their efficacy.
Q: How often should a temperature log be recorded?
A: A temperature log should be recorded at regular intervals, typically every day or every shift, to monitor temperature fluctuations and identify any deviations.
Q: What temperature range is recommended for storing vaccines and biologics?
A: The recommended temperature range for storing vaccines and biologics is usually between 2°C to 8°C (36°F to 46°F).
Q: What should be done if a temperature deviation is detected?
A: If a temperature deviation is detected, it is important to take immediate action, such as transferring the vaccines or biologics to a correctly temperature-controlled storage unit and reporting the incident.
Q: Who is responsible for maintaining the temperature log?
A: The person or team responsible for handling vaccines and biologics, such as healthcare professionals or vaccine coordinators, are typically responsible for maintaining the temperature log.
Q: Can temperature logs be stored electronically?
A: Yes, temperature logs can be stored electronically using specialized software or digital monitoring systems.
Q: How long should temperature logs be retained?
A: Temperature logs should be retained for a specified period of time, as per regulatory requirements, typically for at least 3 to 5 years.
Q: What are the consequences of improper temperature storage?
A: Improper temperature storage can lead to a loss of potency or efficacy of vaccines and biologics, compromising their effectiveness in preventing or treating diseases.