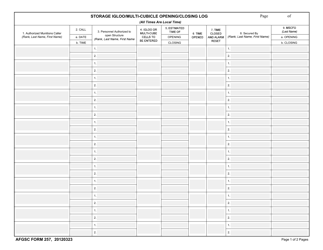
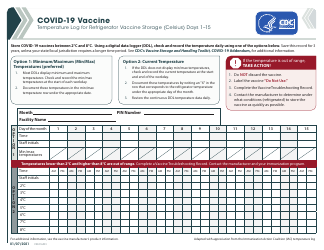
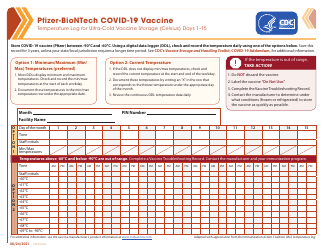
Form 52504 Vaccine Storage Temperature Log (Fahrenheit) - Minnesota
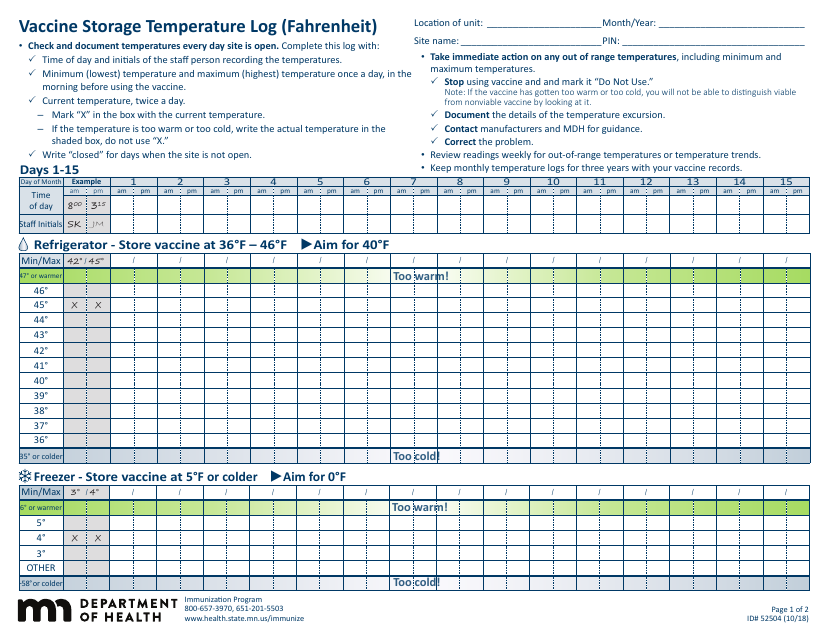
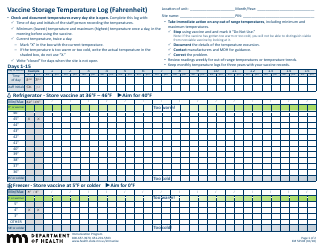
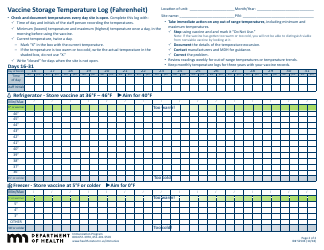
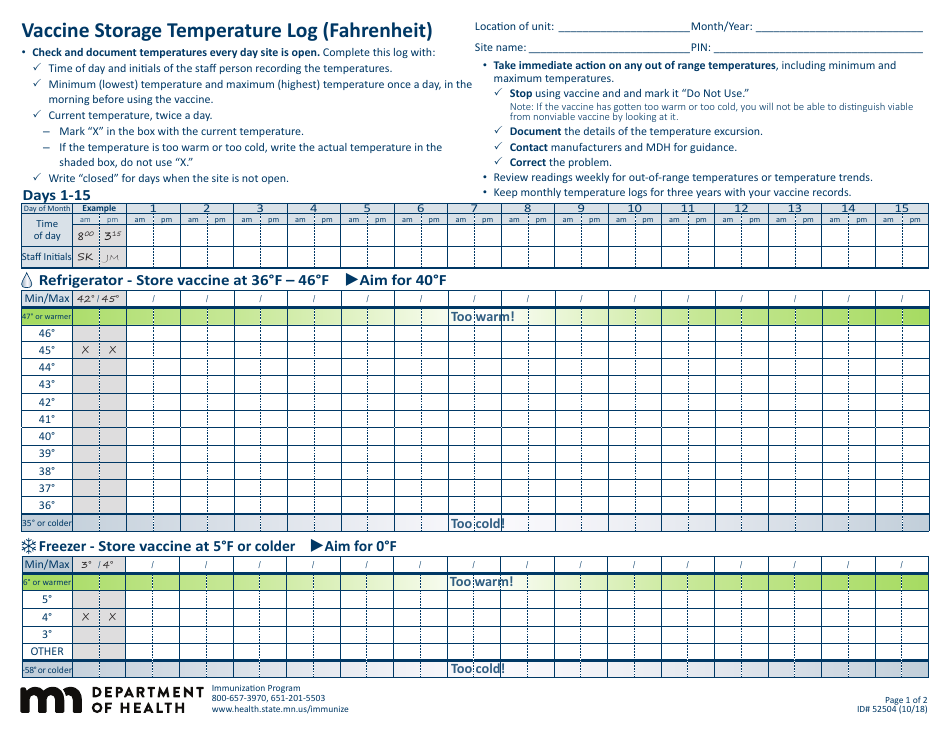
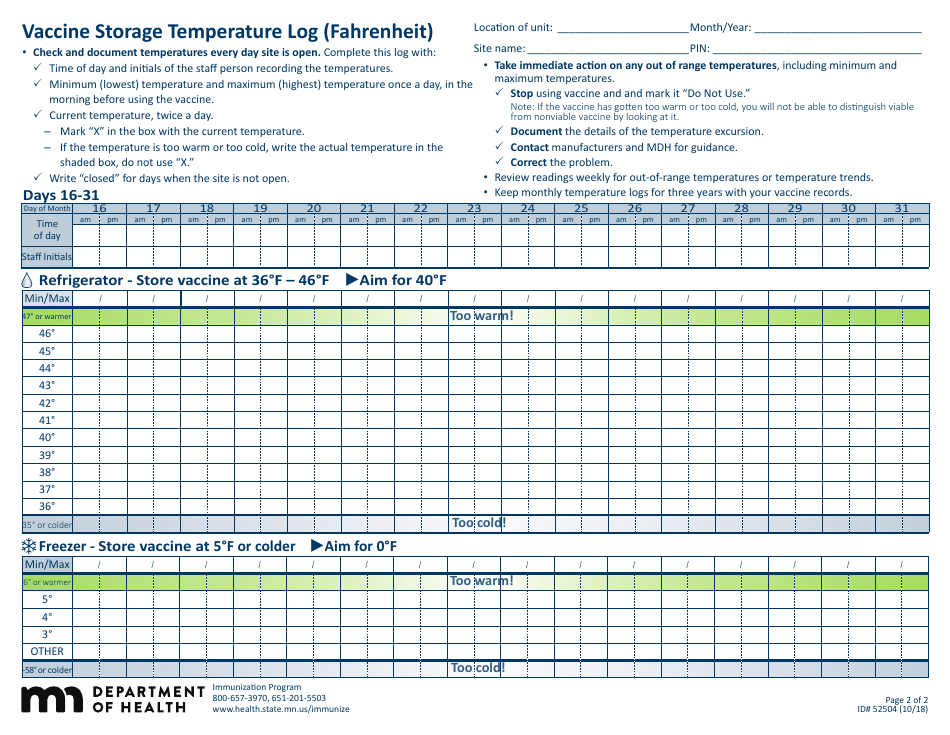
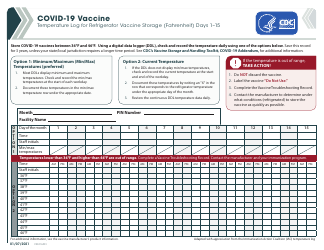
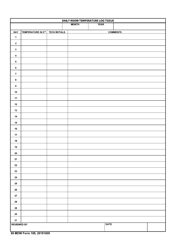
Form 52504, referred to as the Vaccine Storage Temperature Log (Fahrenheit) - Minnesota, is used by health facilities in Minnesota to track and record the temperature of their vaccine storage units. It helps in ensuring that vaccines are stored within the proper temperature range to maintain their effectiveness. This form includes areas to record the minimum and maximum temperatures for each day, allowing staff to identify any temperature excursions that could potentially affect the potency of stored vaccines.
The Form 52504 Vaccine Storage Temperature Log (Fahrenheit) - Minnesota is typically filed by healthcare facilities and providers in the state of Minnesota who manage and distribute vaccines. This includes hospitals, clinics, pharmacies, and possibly schools or other organizations where vaccinations are administered. The purpose of this form is to track and ensure that vaccines are stored at the appropriate temperatures as per the guidelines, to maintain their efficacy.
FAQ
Q: What is the Form 52504 Vaccine Storage Temperature Log?
A: The Form 52504 Vaccine Storage Temperature Log is a document used in Minnesota for recording the temperature of the area where vaccines are stored. It helps ensure vaccines are kept at the correct temperature to maintain their efficacy.
Q: What temperature units does Form 52504 use?
A: Form 52504 records temperatures in Fahrenheit.
Q: Why is it important to monitor vaccine storage temperatures?
A: Monitoring vaccine storage temperatures is important to ensure the efficacy of the vaccines. If a vaccine is stored at an incorrect temperature, it can result in the vaccine losing its potency, and ultimately, its effectiveness in preventing disease.
Q: Who needs to fill out a vaccine storage temperature log?
A: Health professionals and staff who handle and store vaccines, particularly in clinics, hospitals, and pharmacies, need to fill out a vaccine storage temperature log.