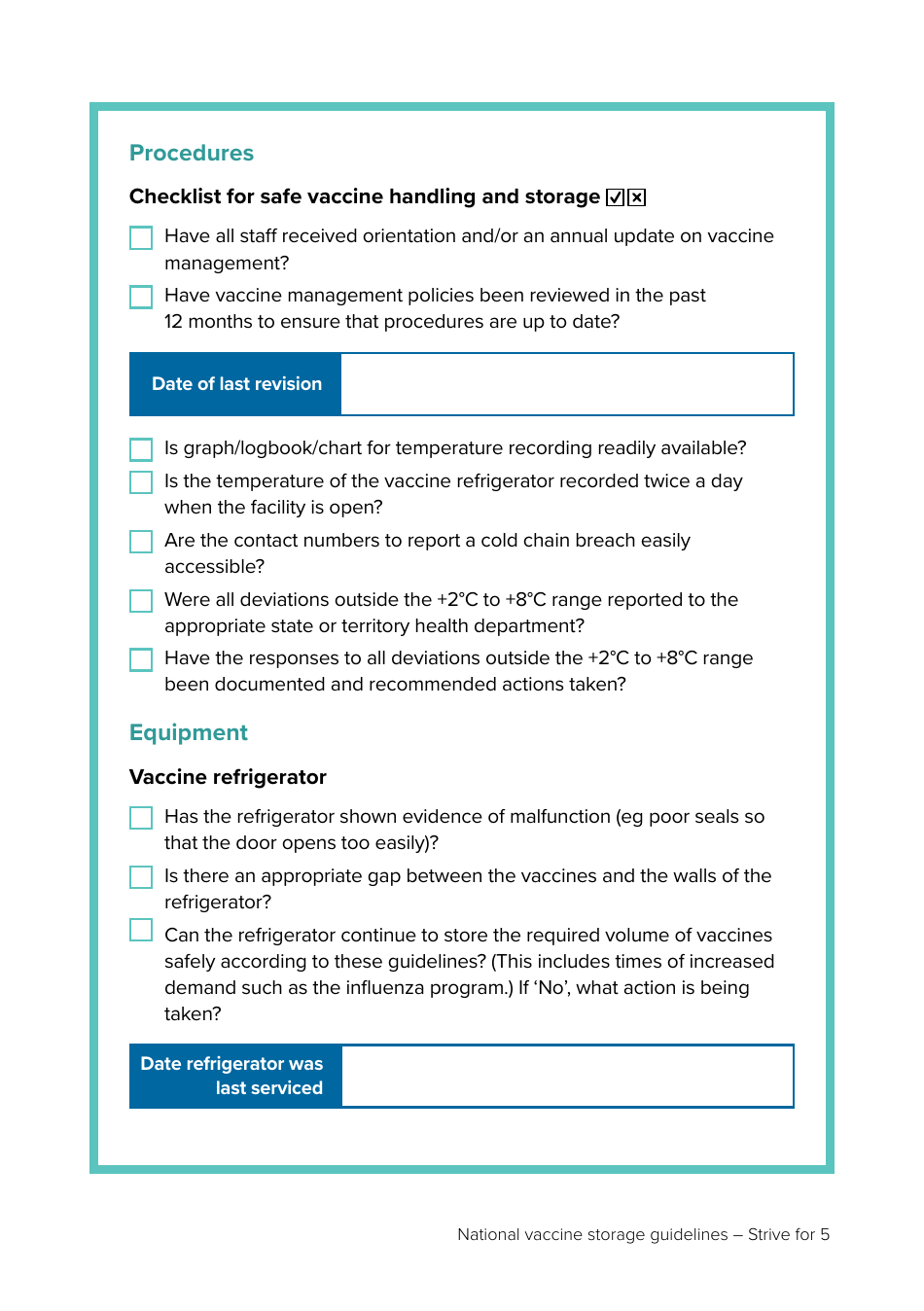
Appendix 2 Vaccine Storage Self-audit - Australia
The Appendix 2 Vaccine Storage Self-audit in Australia is a document used to regularly review and monitor the storage conditions of vaccines. The document includes checking the refrigerator temperature logs, ensuring vaccines are stored within recommended temperature range, the vaccines haven't expired, and the fridge is not overcrowded. This document is crucial in maintaining the potency of vaccines, ensuring they are stored correctly, and implementing corrective actions if any issues are found during the audit.
The Appendix 2 Vaccine Storage Self-audit document in Australia is usually filed by healthcare facilities that store and administer vaccines. This predominantly includes hospitals, general practitioners' clinics, pharmacies, and other health centres that participate in the National Immunisation Program. The self-audit form is part of Australia’s commitment to ensure vaccines' potency and safety by maintaining a level of quality control over vaccine storage and handling practices.
FAQ
Q: What is the Vaccine Storage Self-audit in Australia?
A: The Vaccine Storage Self-audit in Australia is a system implemented to ensure that vaccines are stored under optimal conditions to maintain their effectiveness. It involves regular checks on the storage system to avoid mishandling, improper storage, and temperature fluctuations.
Q: How often is a Vaccine Storage Self-audit conducted in Australia?
A: Though the frequency may vary depending on different jurisdictions and organizations, it's usually recommended that vaccine storage self-audits are conducted annually in Australia.
Q: Why is the Vaccine Storage Self-audit in Australia important?
A: The Vaccine Storage Self-audit is important in Australia as it ensures the efficacy of stored vaccines. If vaccines are improperly stored, they can lose their effectiveness, which can compromise their ability to protect individuals from diseases. Thus, regular audits help maintain the quality of vaccinations.
Q: Who is responsible for Vaccine Storage Self-audit in Australia?
A: The responsibility for conducting a Vaccine Storage Self-audit in Australia typically lies with the relevant health departments, healthcare providers, and vaccine service providers, as they directly handle vaccine storage.
Q: What does a Vaccine Storage Self-audit in Australia involve?
A: A Vaccine Storage Self-audit in Australia involves checking the physical condition of the fridge where vaccines are stored, monitoring and recording the fridge temperature at least twice a day, ensuring the fridge is at the correct temperature, checking the organisation of vaccines in the fridge, and maintaining the emergency vaccine management plan among others.