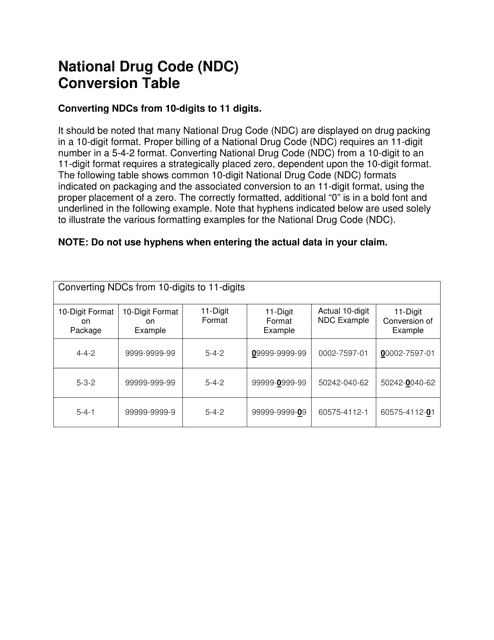
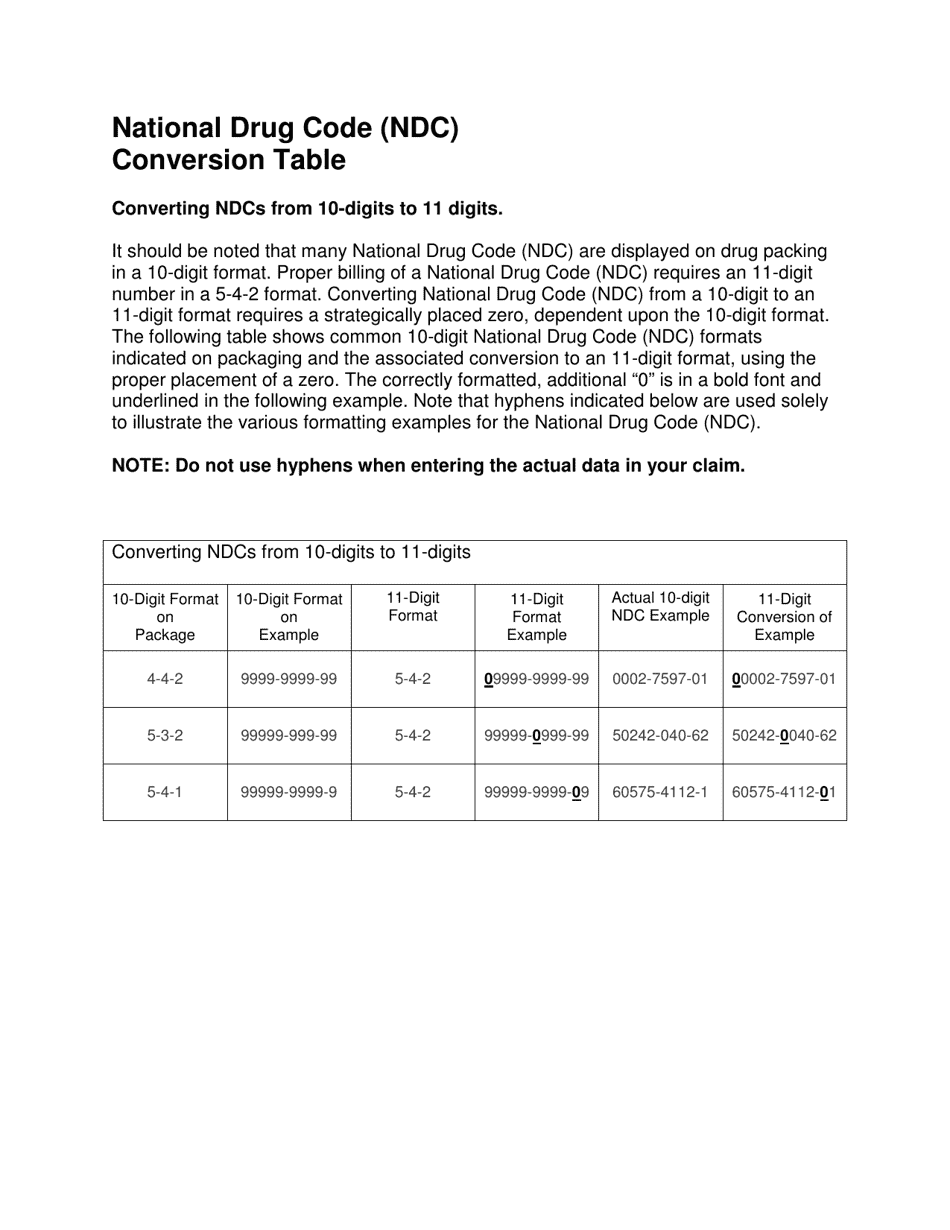
National Drug Code (Ndc) Conversion Table
The National Drug Code (NDC) Conversion Table is used to convert NDC numbers from one format to another. It helps ensure consistency and accuracy when listing and identifying drugs in various healthcare systems and documentation.
The National Drug Code (NDC) Conversion Table is not filed by any specific entity. It is maintained by the U.S. Food and Drug Administration (FDA) and is accessible to the public.
FAQ
Q: What is the National Drug Code (NDC)?
A: The National Drug Code (NDC) is a unique identifier for medications in the United States.
Q: What is the purpose of the NDC?
A: The purpose of the NDC is to provide a standardized and unique identifier for medications, which helps in the identification and tracking of drugs.
Q: What information does the NDC provide?
A: The NDC provides information about the manufacturer, product, and package size of a medication.
Q: Why is the NDC important?
A: The NDC is important for various purposes, such as identifying and tracking medications, ensuring patient safety, and facilitating accurate billing and reimbursement.
Q: What is the NDC conversion table?
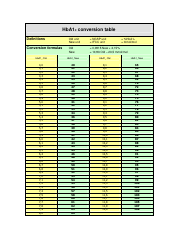
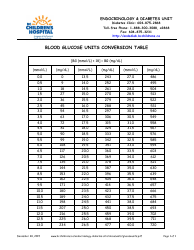
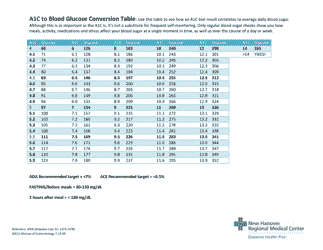
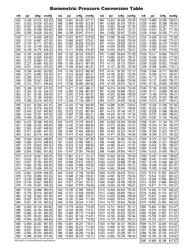
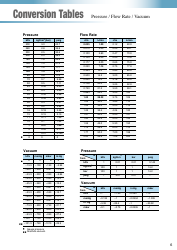
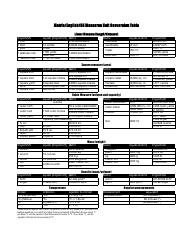
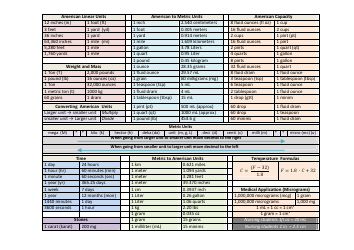
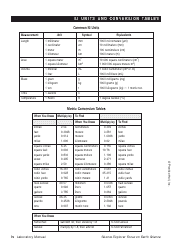
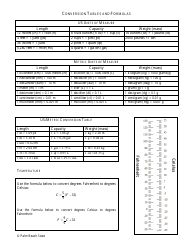
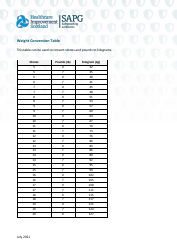
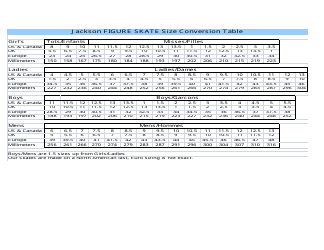
A: The NDC conversion table is a reference table that lists the NDCs of medications and their corresponding codes in different formats, such as the NDC11, NDC10, and NDC9.
Q: What is the purpose of the NDC conversion table?
A: The purpose of the NDC conversion table is to facilitate the translation of NDCs between different formats, enabling efficient data exchange between healthcare systems and organizations.
Q: Is the NDC conversion table regularly updated?
A: Yes, the NDC conversion table is regularly updated to ensure accuracy and reflect any changes in NDCs for medications.