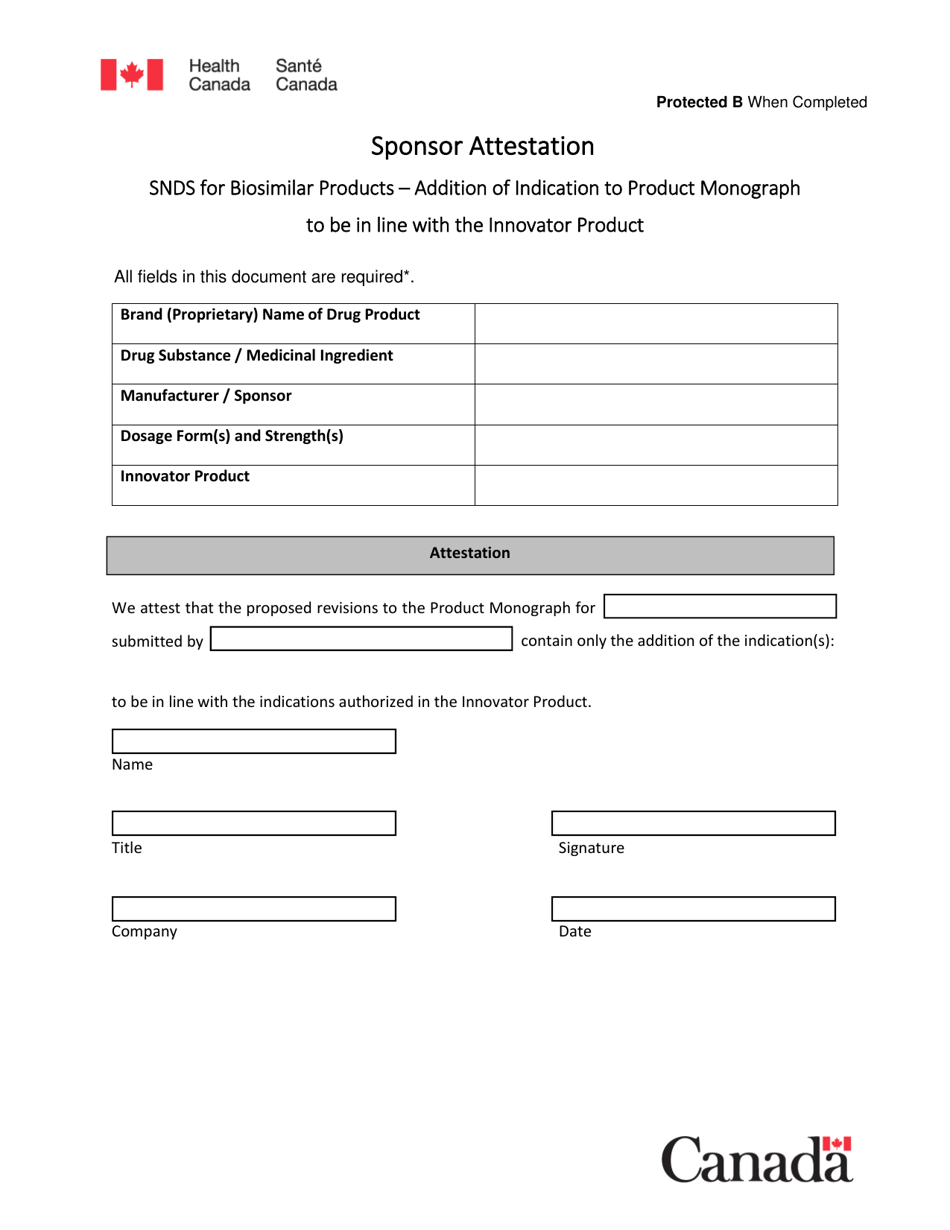
Sponsor Attestation: Snds for Biosimilar Products - Addition of Indication to Product Monograph to Be in Line With the Innovator Product - Canada
The sponsor files the Sponsor Attestation for Biosimilar Products - Addition of Indication to Product Monograph to be in line with the innovator product in Canada.
FAQ
Q: What is the Sponsor Attestation?
A: The Sponsor Attestation is a requirement for biosimilar products in Canada.
Q: What does SNDs stand for?
A: SNDs stands for Submission for New Drug.
Q: What is a biosimilar product?
A: A biosimilar product is a highly similar version of an approved biological reference product.
Q: What does the addition of indication to product monograph mean?
A: It means updating the information about the approved uses of the product.
Q: Why does the addition of indication need to be in line with the innovator product?
A: To ensure consistency and accuracy in the information provided to healthcare professionals and patients.
Q: Is the Sponsor Attestation required for all biosimilar products?
A: Yes, the Sponsor Attestation is required for all biosimilar products in Canada.