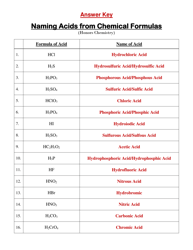
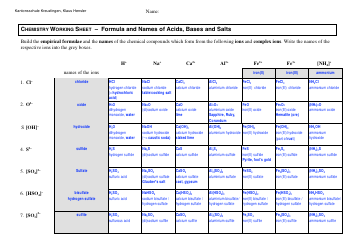
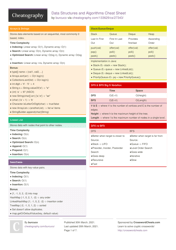
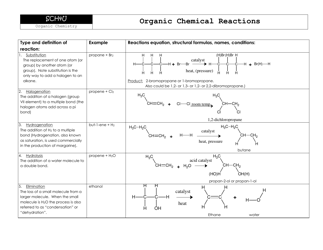
Chemistry Cheat Sheet - Lewis Structures
A Chemistry Cheat Sheet on Lewis Structures is a quick reference guide that helps students understand and draw Lewis structures, which are diagrams that show the arrangement of atoms and their bonds in a molecule. It is used to study and understand the structural properties of various chemical compounds.
FAQ
Q: What are Lewis structures?
A: Lewis structures are diagrams that show the arrangement of atoms and bonds within a molecule.
Q: How do you draw Lewis structures?
A: To draw Lewis structures, you start by counting the total number of valence electrons in the molecule, then arrange the atoms and bonds while satisfying the octet rule.
Q: What is the octet rule?
A: The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a full outer electron shell with 8 electrons.
Q: What is a valence electron?
A: Valence electrons are the electrons in the outermost energy level of an atom. They influence the chemical properties and reactivity of the atom.
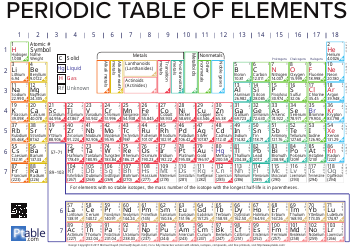
Q: How do you determine the number of valence electrons?
A: The number of valence electrons for main group elements can be determined by looking at their group number on the periodic table.
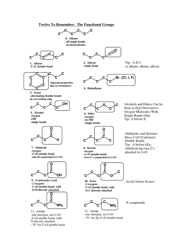
Q: What is a lone pair?
A: A lone pair is a pair of valence electrons that are not involved in bonding and are localized on a specific atom.
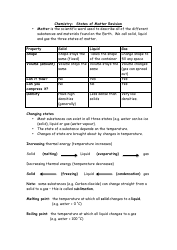
Q: What are double and triple bonds?
A: Double bonds consist of two pairs of shared electrons between two atoms, while triple bonds consist of three pairs of shared electrons.
Q: What is the significance of Lewis structures?
A: Lewis structures help to visualize and understand the bonding and structure of molecules, and they provide a framework for predicting molecular properties and reactions.