Chemistry Cheat Sheet - Acids
A Chemistry Cheat Sheet - Acids is a concise reference guide that provides information about various acids and their properties. It is used to quickly review and understand important concepts related to acids in the field of chemistry.
FAQ
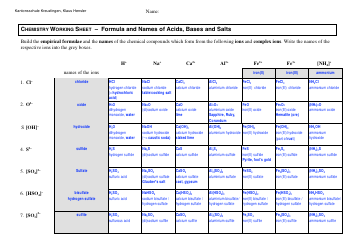
Q: What are acids?
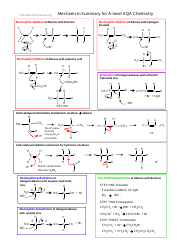
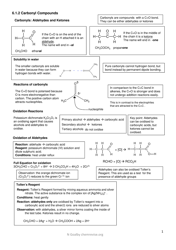
A: Acids are substances that donate hydrogen ions in a chemical reaction.
Q: How can acids be identified?
A: Acids can be identified by their sour taste, ability to dissolve certain metals, and their ability to turn blue litmus paper red.
Q: What is the pH scale?
A: The pH scale is a measure of how acidic or basic a substance is. It ranges from 0 to 14, with 0 being the most acidic and 14 being the most basic.
Q: What are some common examples of acids?
A: Some common examples of acids include vinegar (acetic acid), lemon juice (citric acid), and battery acid (sulfuric acid).
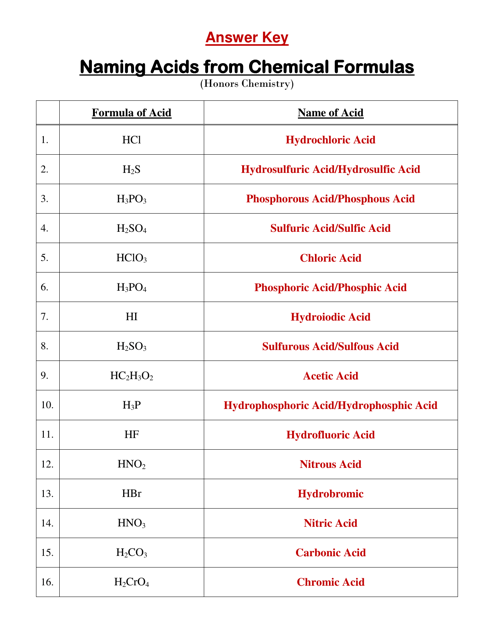
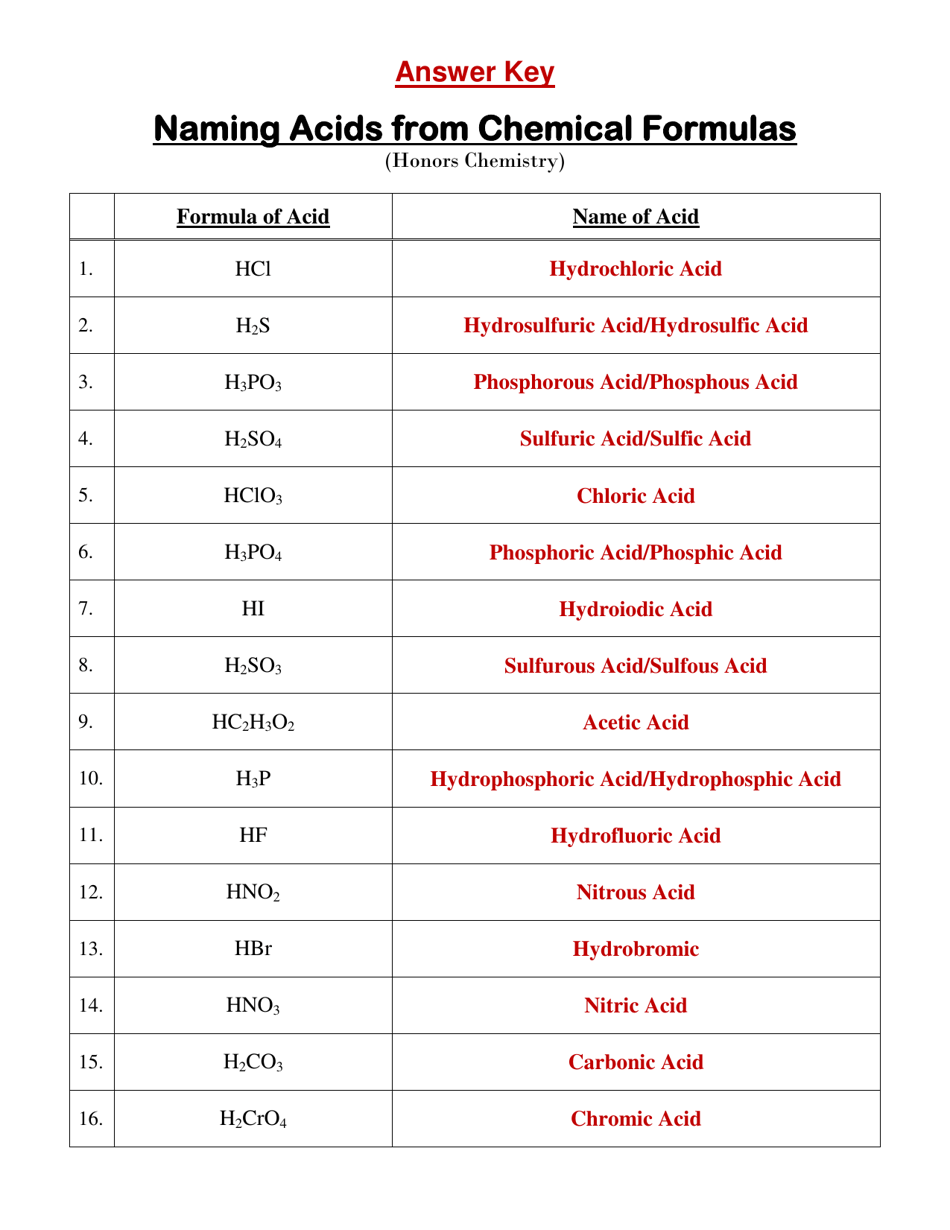
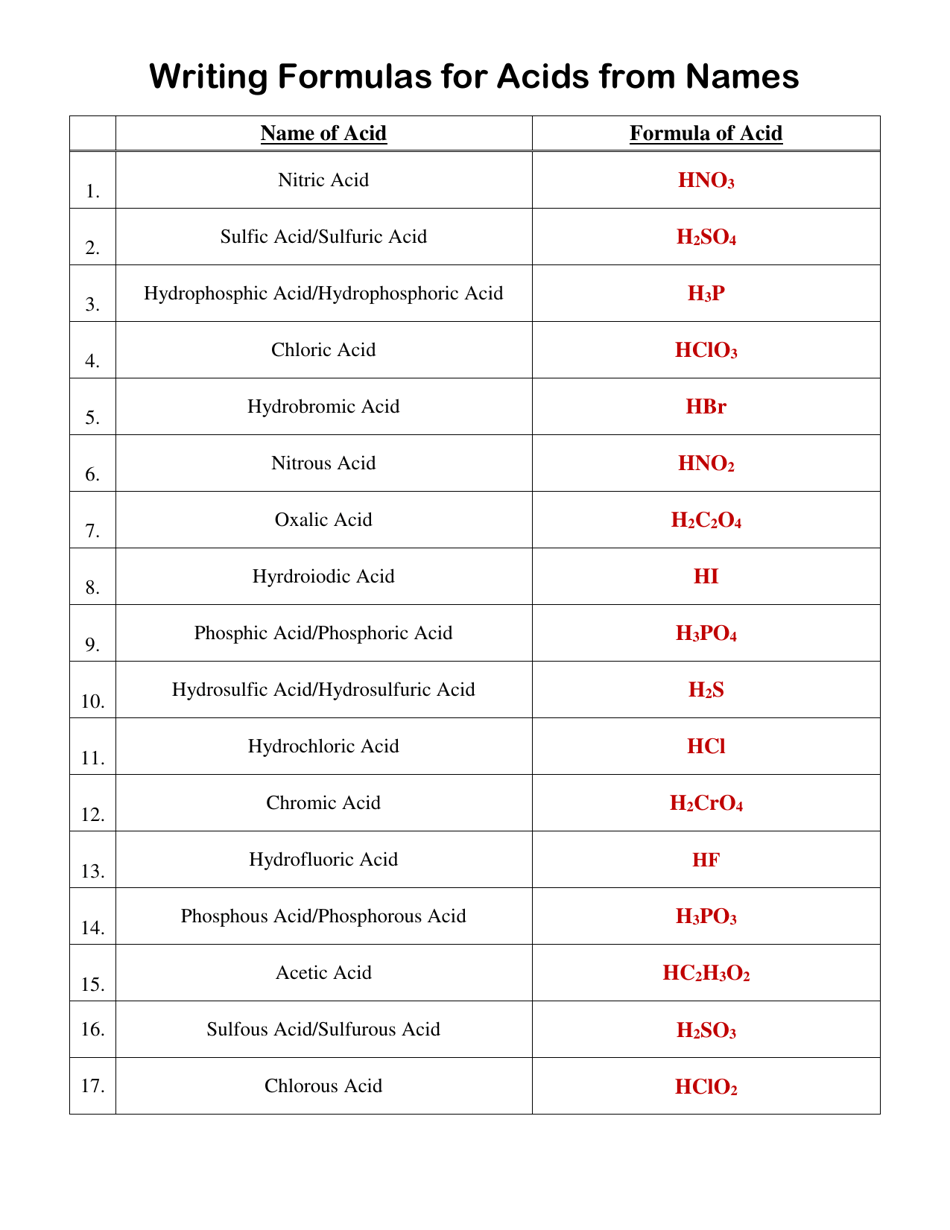
Q: What is the chemical formula for hydrochloric acid?
A: The chemical formula for hydrochloric acid is HCl.
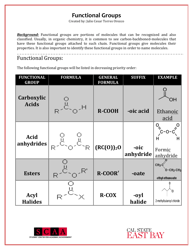
Q: What are the properties of acids?
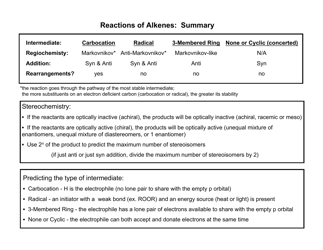
A: Acids have a pH less than 7, react with bases to form salts and water, and can corrode metals.
Q: What is the role of acids in the human body?
A: Acids play a key role in digestion, as the stomach produces hydrochloric acid to help break down food.
Q: How are acids used in industry?
A: Acids are used in a variety of industrial processes, such as metal cleaning, pH regulation, and manufacturing of various chemicals.
Q: What are some safety precautions when dealing with acids?
A: Some safety precautions include wearing protective clothing and eyewear, handling acids in a well-ventilated area, and avoiding contact with skin or eyes.
Q: Can acids be harmful to the environment?
A: Yes, some acids can be harmful to the environment if not properly managed. They can contaminate water sources and disrupt ecosystems.