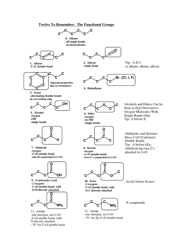
Chemical Bonding Cheat Sheet
A Chemical Bonding Cheat Sheet is a document that provides a concise summary of the important concepts and information related to chemical bonding. It is designed to help students and individuals easily understand and remember the key principles and patterns involved in chemical bonding.
FAQ
Q: What is a chemical bond?
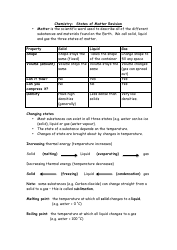
A: A chemical bond is a force of attraction between atoms that holds them together in a chemical compound.
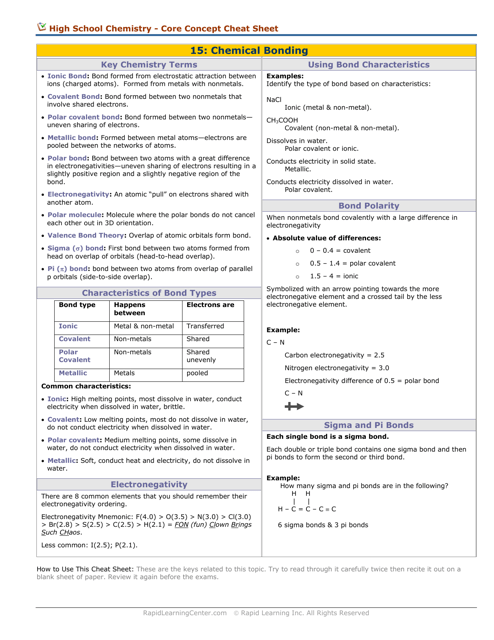
Q: What are the three main types of chemical bonds?
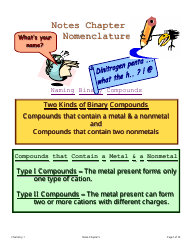
A: The three main types of chemical bonds are ionic bonds, covalent bonds, and metallic bonds.
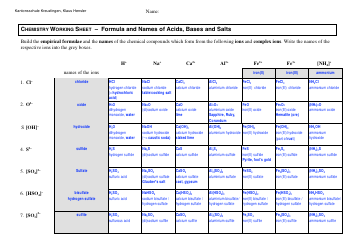
Q: What is an ionic bond?
A: An ionic bond is a bond formed between two oppositely charged ions.
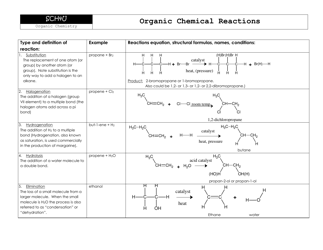
Q: What is a covalent bond?
A: A covalent bond is a bond formed by the sharing of electrons between atoms.
Q: What is a metallic bond?
A: A metallic bond is a bond formed by the attraction of positive metal ions to delocalized electrons.
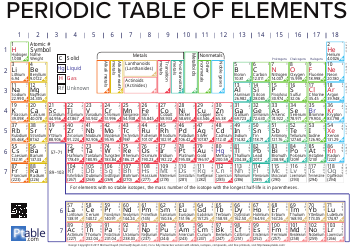
Q: What is electronegativity?
A: Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond.
Q: What is a polar bond?
A: A polar bond is a covalent bond in which the electrons are not shared equally between atoms.
Q: What is an nonpolar bond?
A: A nonpolar bond is a covalent bond in which the electrons are shared equally between atoms.
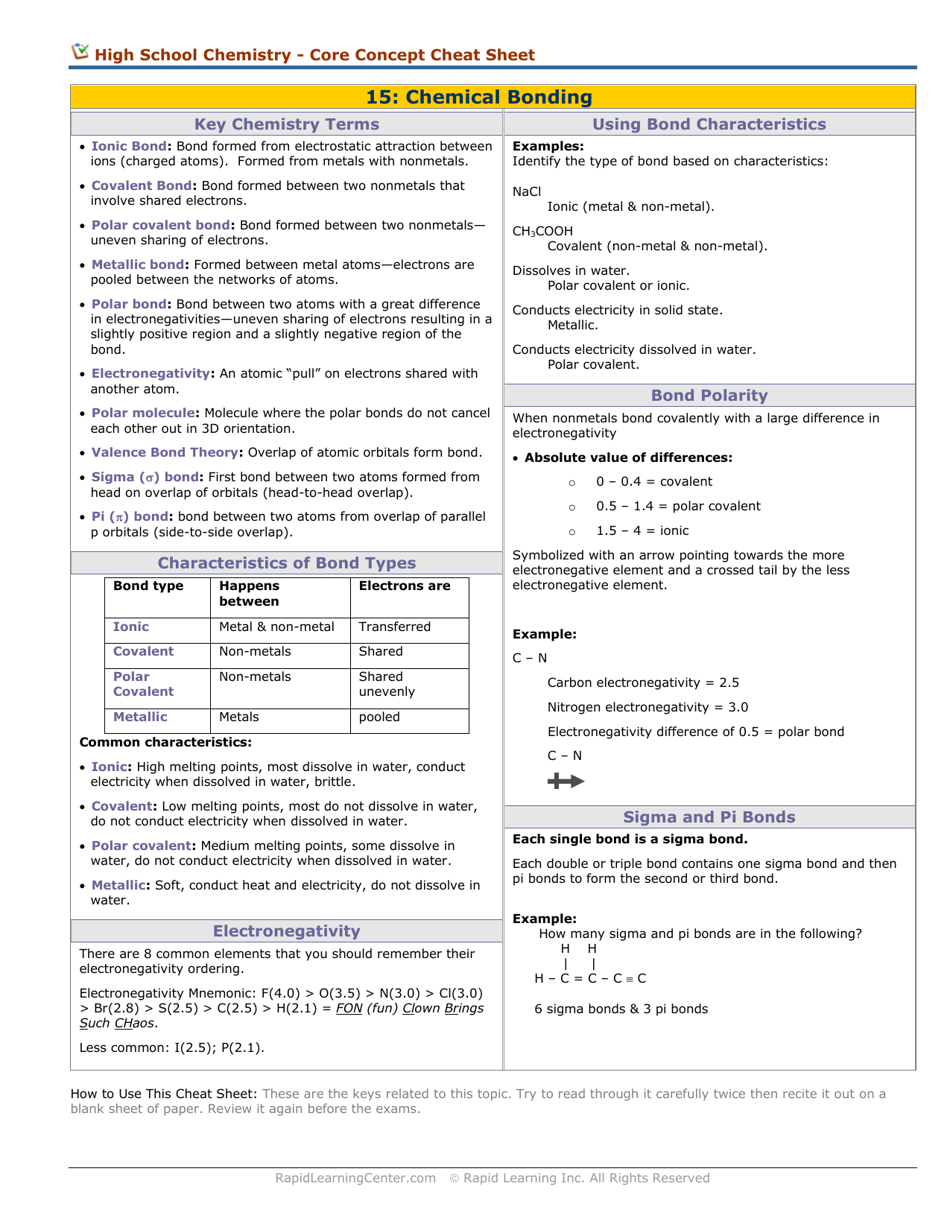
Q: What is a Lewis structure?
A: A Lewis structure is a diagram that shows the bonding between atoms in a molecule and the lone pairs of electrons.
Q: What is hybridization?
A: Hybridization is the process of mixing atomic orbitals to form new hybrid orbitals that are used in bonding.