Chemistry Equations Cheat Sheet - Jackson Taylor
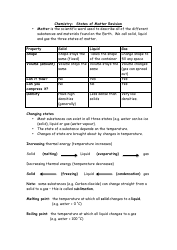
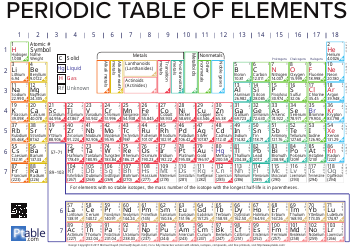
The Chemistry Equations Cheat Sheet by Jackson Taylor is a document that provides a quick reference guide for common chemistry equations. It helps students and learners to quickly find and understand important chemical equations for various concepts in chemistry.
FAQ
Q: What is a chemical equation?
A: A representation of a chemical reaction using chemical symbols and formulas.
Q: What is a balanced chemical equation?
A: An equation that has the same number of atoms of each element on both sides.
Q: How do you balance a chemical equation?
A: By adjusting the coefficients in front of the chemical formulas to equalize the number of atoms on both sides.
Q: What does the arrow in a chemical equation represent?
A: The direction of the chemical reaction, from reactants to products.
Q: What are reactants?
A: The substances that are present before a chemical reaction occurs.
Q: What are products?
A: The substances that are formed as a result of a chemical reaction.
Q: What is a synthesis reaction?
A: A reaction where two or more substances combine to form a more complex product.
Q: What is a decomposition reaction?
A: A reaction where a compound breaks down into simpler substances.
Q: What is a combustion reaction?
A: A reaction where a substance reacts with oxygen, releasing energy in the form of heat and light.
Q: What is a single replacement reaction?
A: A reaction where one element replaces another element in a compound to form a new compound and a different element.