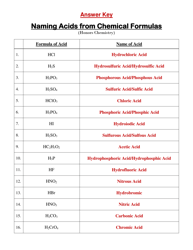
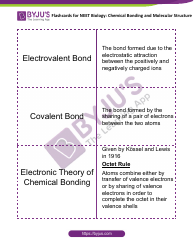
Ap Chemistry Polyatomic List
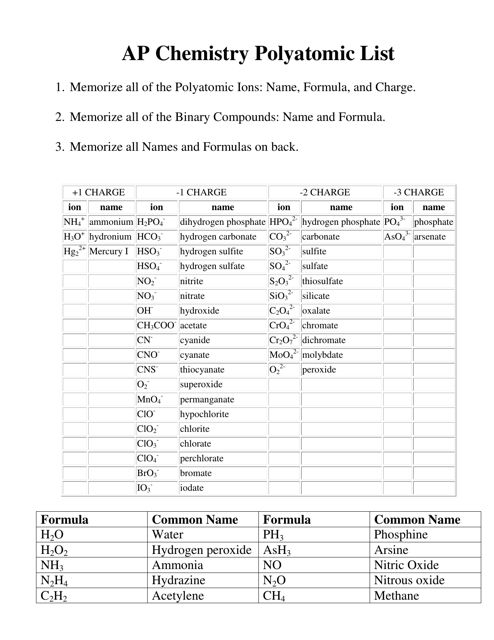
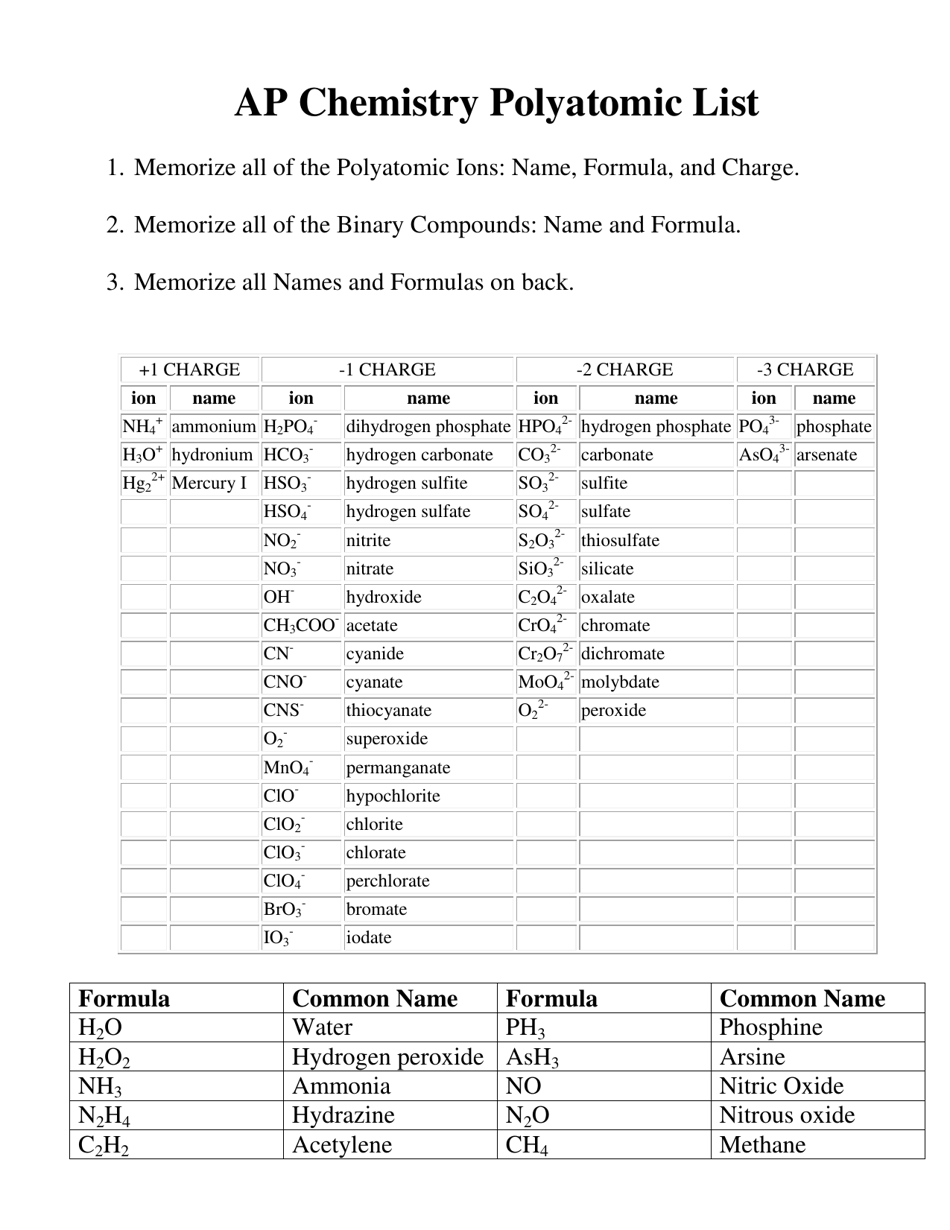
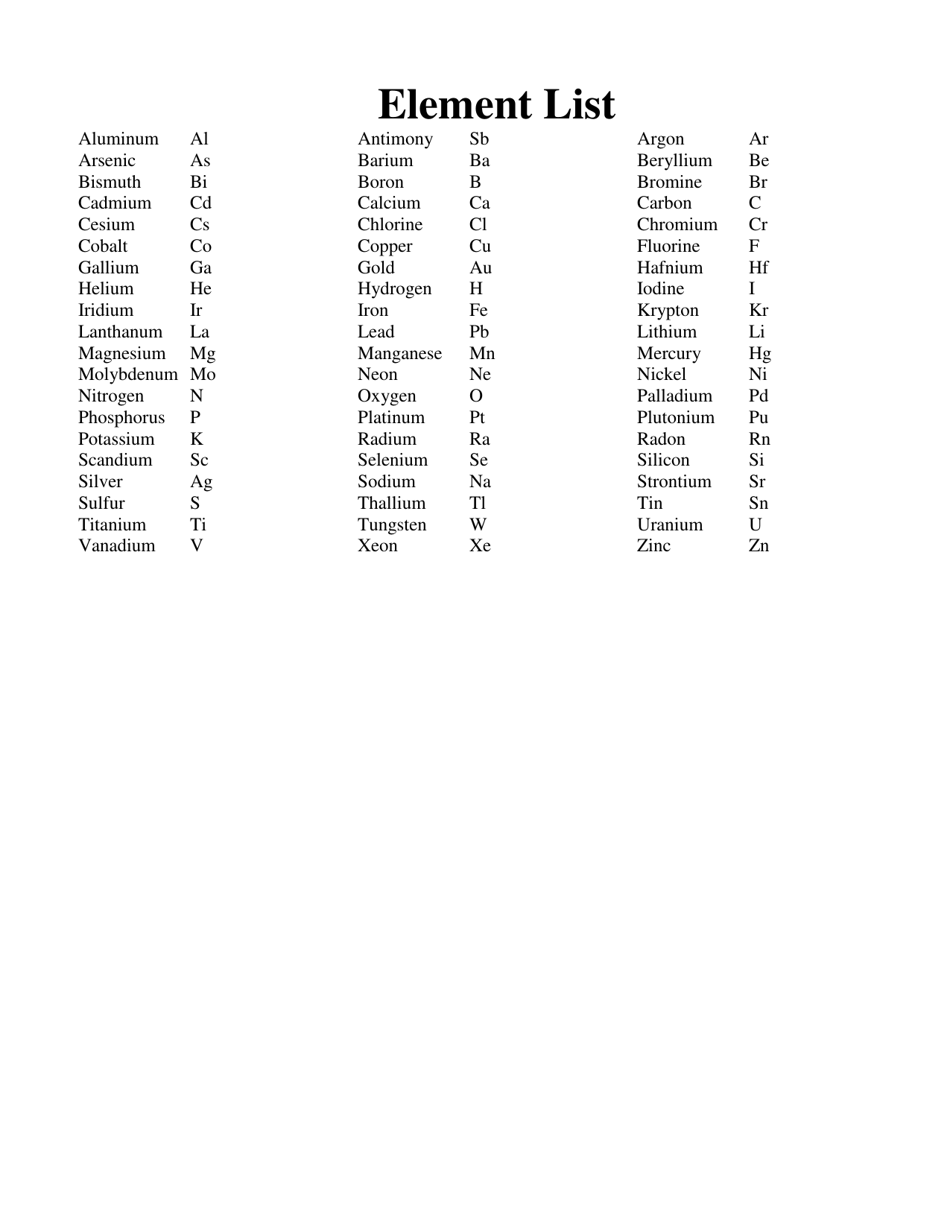
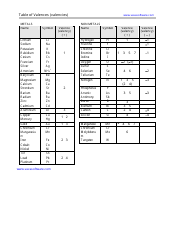
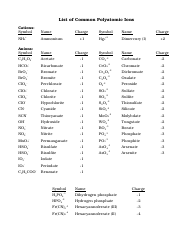
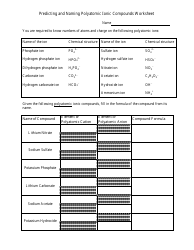
The AP Chemistry Polyatomic List is a reference guide for students studying Advanced Placement (AP) Chemistry. It lists the names, formulas, and charges of commonly encountered polyatomic ions. These ions are important for understanding chemical reactions and formulas.
The AP Chemistry polyatomic list is typically provided by the College Board, the organization that administers the AP exams.
FAQ
Q: What is a polyatomic ion?
A: A polyatomic ion is a charged particle composed of two or more atoms.
Q: What is the difference between a polyatomic ion and a simple ion?
A: A polyatomic ion is composed of multiple atoms, while a simple ion is composed of just a single atom.
Q: Why are polyatomic ions important in chemistry?
A: Polyatomic ions play a crucial role in balancing chemical equations and determining the overall charge of compounds.
Q: What are some examples of common polyatomic ions?
A: Some common polyatomic ions include nitrate (NO3-), carbonate (CO3^2-), sulfate (SO4^2-), and ammonium (NH4+).
Q: How can you identify a polyatomic ion in a chemical formula?
A: Polyatomic ions are typically enclosed in parentheses and have a superscript that indicates the charge.