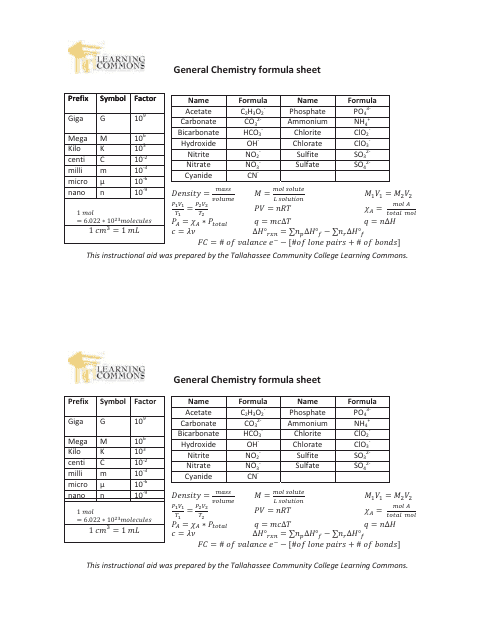
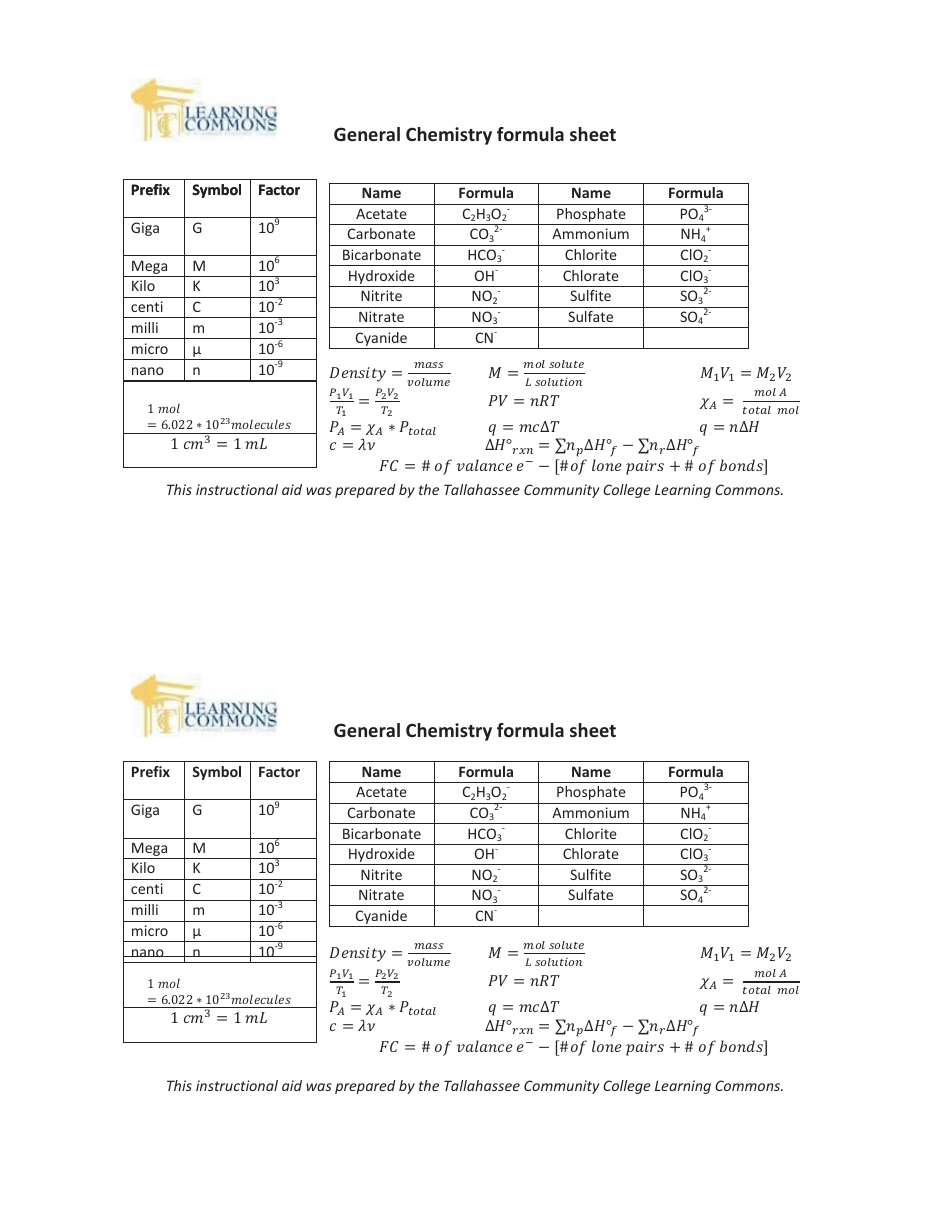
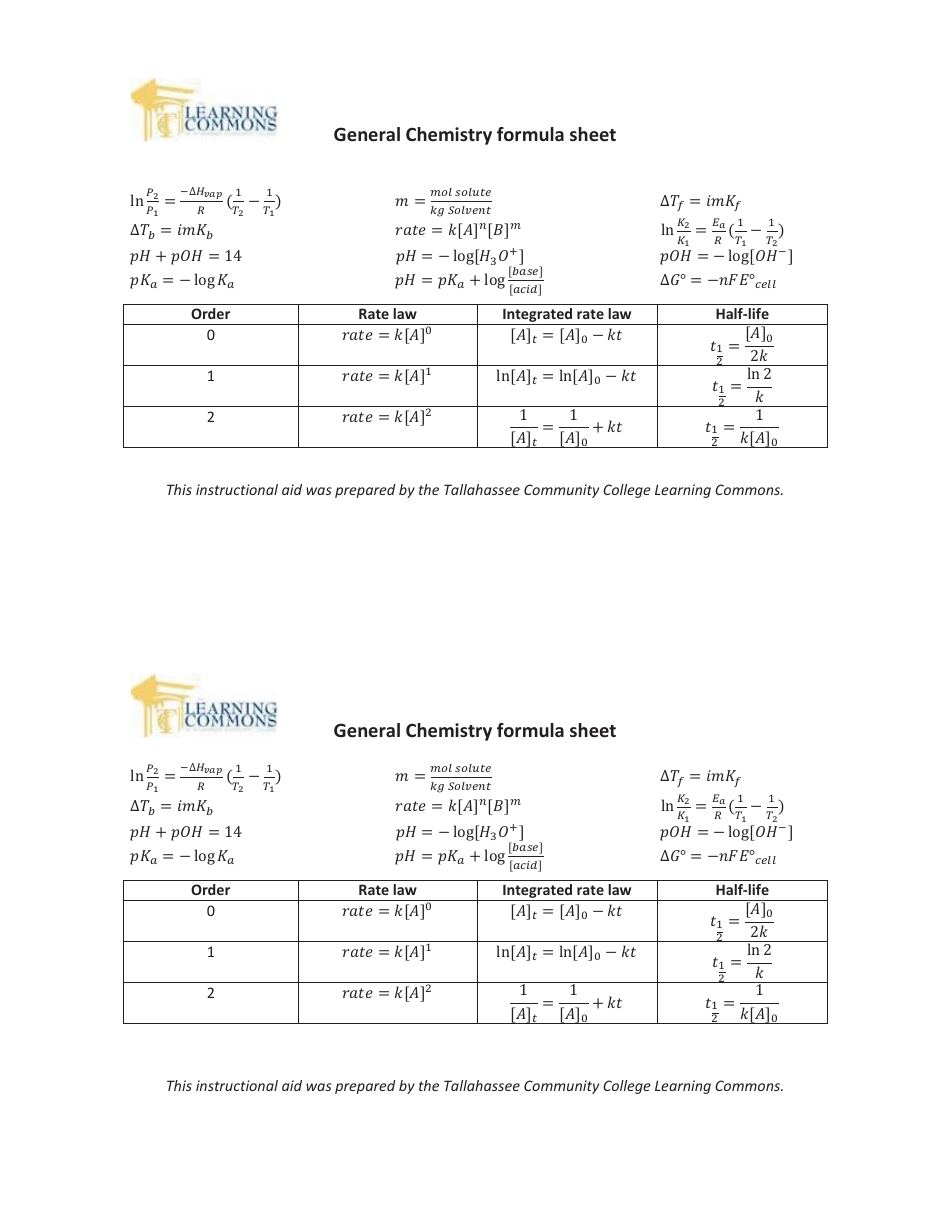
General Chemistry Formula Sheet
The General Chemistry Formula Sheet is a document that contains important formulas and equations used in the study of general chemistry. It serves as a quick reference guide for students and professionals to easily access the formulas needed for calculations and problem-solving in the field of chemistry.
The general chemistry formula sheet can be filed by teachers, students, or educational institutions.
FAQ
Q: What is a formula sheet?
A: A formula sheet is a condensed collection of important formulas and concepts in a particular subject.
Q: Why is a formula sheet important?
A: A formula sheet is important because it provides a quick reference for key formulas and equations that are commonly used in a subject, helping students solve problems more efficiently.
Q: What is general chemistry?
A: General chemistry is the introductory branch of chemistry that covers the fundamental concepts and principles of the subject.
Q: What are some common topics covered in general chemistry?
A: Some common topics covered in general chemistry include atomic structure, chemical bonding, stoichiometry, thermodynamics, and acid-base chemistry.
Q: What are some important formulas in general chemistry?
A: Some important formulas in general chemistry include the ideal gas law (PV = nRT), the pH formula (pH = -log[H+]), and the formula for calculating molarity (M = moles of solute / liters of solution).
Q: What is the ideal gas law?
A: The ideal gas law is a mathematical relationship between the pressure (P), volume (V), number of moles (n), and temperature (T) of a gas. It is expressed as PV = nRT, where R is the gas constant.
Q: What is stoichiometry?
A: Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions.
Q: What is thermodynamics?
A: Thermodynamics is the branch of chemistry that deals with the study of energy and its transformations in chemical and physical systems.
Q: What is acid-base chemistry?
A: Acid-base chemistry is the branch of chemistry that deals with the properties of acids and bases, their reactions, and their effects on each other.
Q: What does pH represent?
A: pH represents the acidity or basicity of a solution. It is a measure of the concentration of hydrogen ions (H+) in a solution.