Ap Chemistry Periodic Table of the Elements
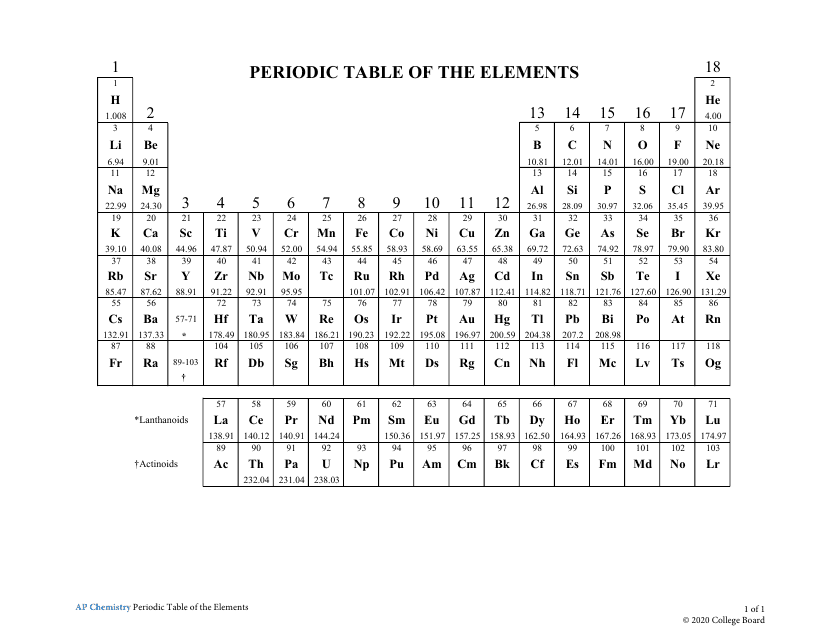
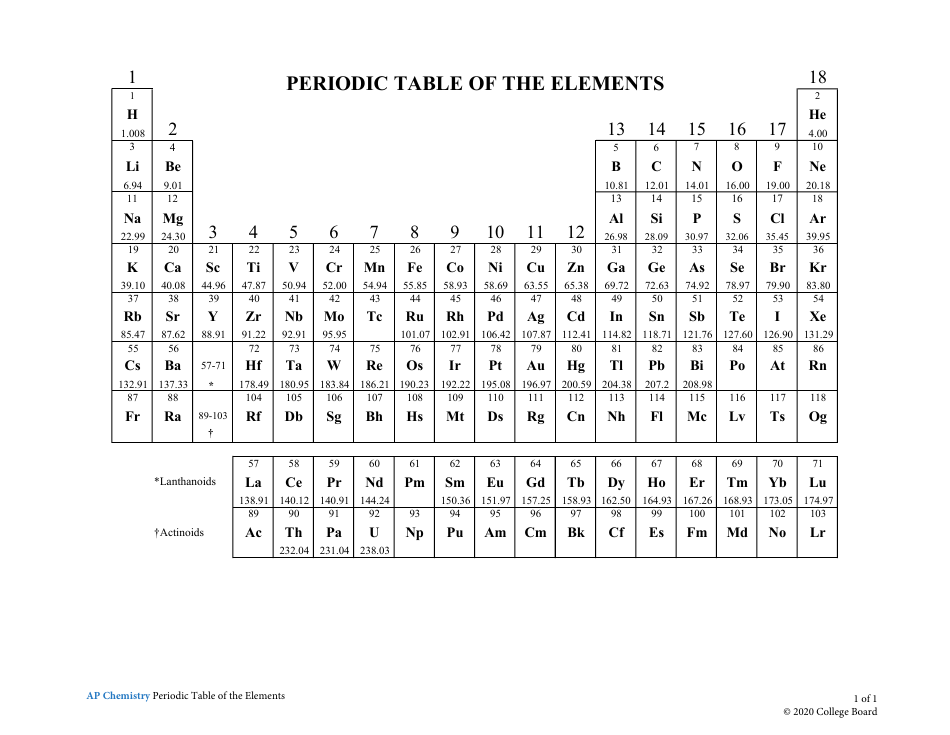
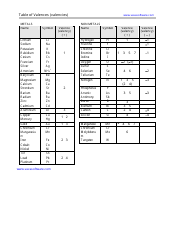
The AP Chemistry Periodic Table of the Elements is a tool used to organize and display information about the known chemical elements. It provides valuable information such as their atomic number, symbol, atomic mass, and other important properties. This periodic table is used specifically in the context of advanced placement (AP) chemistry courses to aid students in studying and understanding the elements and their characteristics.
The Ap Chemistry Periodic Table of the Elements is typically filed by the teachers or instructors responsible for teaching the AP Chemistry course.
FAQ
Q: What is the periodic table of the elements?
A: The periodic table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties.
Q: Why is the periodic table important?
A: The periodic table is important because it provides a systematic way to organize, study, and understand the chemical elements and their properties.
Q: How many elements are in the periodic table?
A: Currently, there are 118 known elements in the periodic table.
Q: What is the atomic number of an element?
A: The atomic number of an element is the number of protons in the nucleus of an atom.
Q: What is the symbol for an element?
A: The symbol for an element is a short, one- or two-letter abbreviation used to represent the element.
Q: What are some examples of elements?
A: Examples of elements include hydrogen, oxygen, carbon, nitrogen, and gold.
Q: What are periods and groups in the periodic table?
A: Periods are horizontal rows, and groups (or families) are vertical columns in the periodic table.
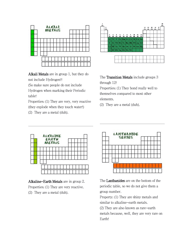
Q: What are some characteristics of metals?
A: Metals are typically shiny, good conductors of electricity and heat, malleable, and ductile.
Q: What are some characteristics of nonmetals?
A: Nonmetals are generally poor conductors of electricity and heat, and they can be brittle.
Q: What are metalloids?
A: Metalloids are elements that have properties intermediate between metals and nonmetals.