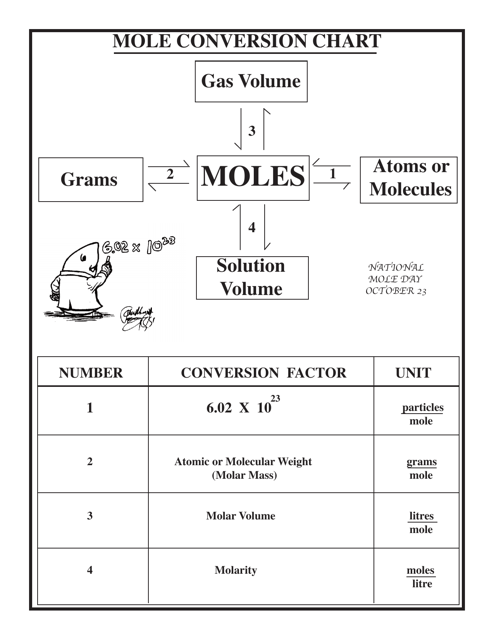
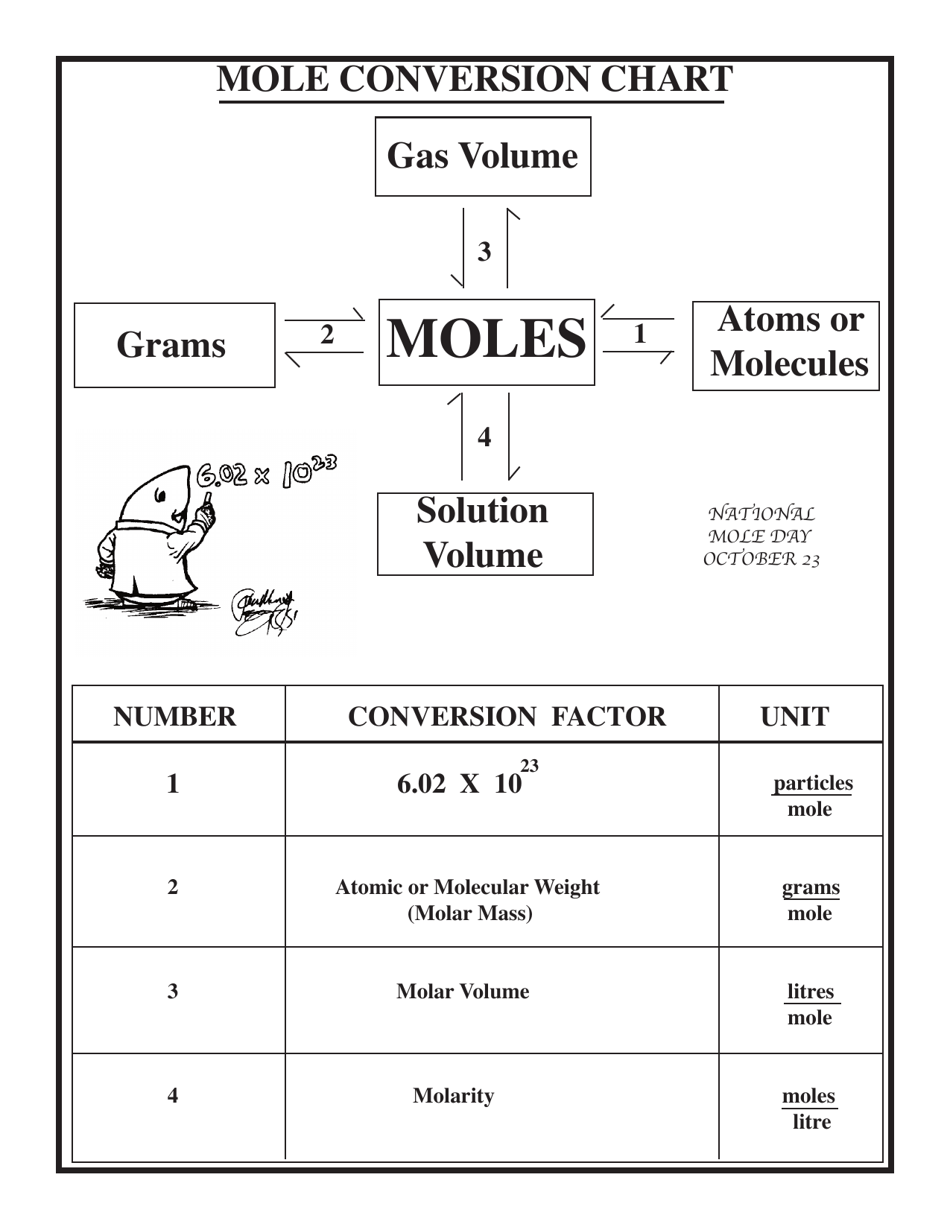
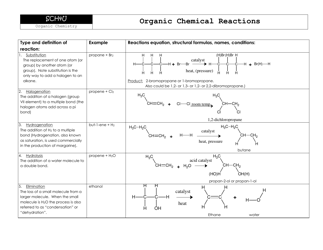
Chemistry Cheat Sheet - Mole Conversion Chart
The Chemistry Cheat Sheet - Mole Conversion Chart is a tool used to help students or individuals convert between different units of measurement in chemistry, particularly when dealing with moles and chemical equations. It provides a quick reference guide for converting from moles to grams, liters, or particles, and vice versa.
FAQ
Q: What is a mole?
A: A mole is a unit used to measure the amount of a substance.
Q: How many particles are in one mole?
A: One mole contains 6.022 x 10^23 particles.
Q: How do you convert moles to grams?
A: To convert moles to grams, multiply the number of moles by the molar mass of the substance.
Q: How do you convert grams to moles?
A: To convert grams to moles, divide the mass of the substance by its molar mass.
Q: What is Avogadro's number?
A: Avogadro's number is 6.022 x 10^23, which represents the number of particles in one mole.
Q: How do you calculate molar mass?
A: To calculate molar mass, add up the atomic masses of all the atoms in a molecule.
Q: What is the formula for calculating moles?
A: Number of moles = Mass / Molar mass
Q: What are some common molar masses?
A: The molar mass of carbon is 12.01 g/mol, oxygen is 16.00 g/mol, and hydrogen is 1.01 g/mol.
Q: What is stoichiometry?
A: Stoichiometry is the study of the quantitative relationships between reactants and products in a chemical reaction.
Q: What is the mole ratio?
A: The mole ratio is the ratio of moles of one substance to moles of another substance in a balanced chemical equation.