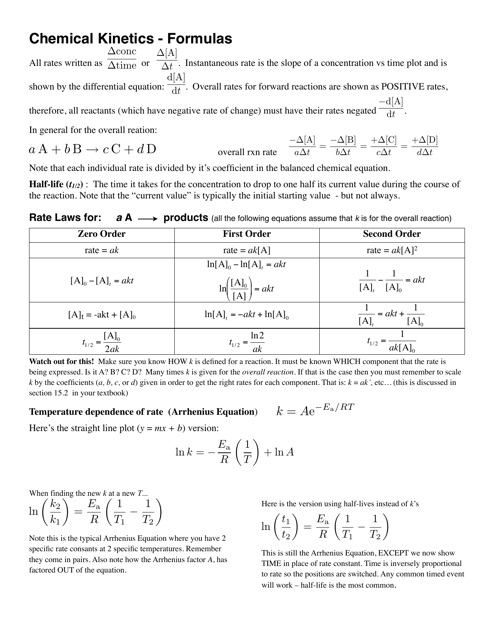
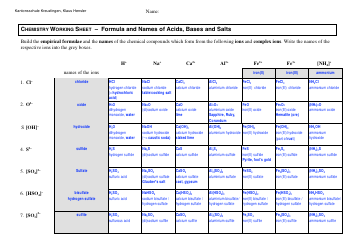
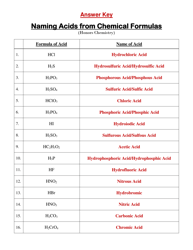
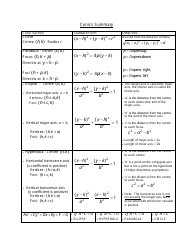
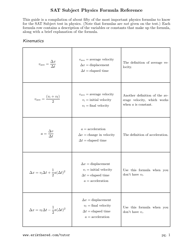
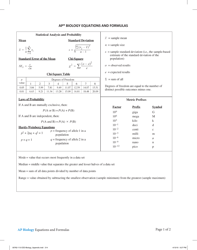
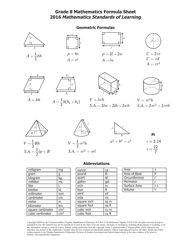
Chemical Kinetics Formulas Cheat Sheet
The Chemical Kinetics Formulas Cheat Sheet is a document that provides a summary of important formulas and equations used in the study of chemical reactions and reaction rates. It serves as a quick reference guide for students and researchers working in the field of chemical kinetics.
FAQ
Q: What is chemical kinetics?
A: Chemical kinetics is the study of the rates of chemical reactions.
Q: What is the rate of a chemical reaction?
A: The rate of a chemical reaction is the change in concentration of a reactant or product per unit of time.
Q: What is the rate law?
A: The rate law is an equation that relates the rate of a reaction to the concentrations of the reactants.
Q: What is the order of a reaction?
A: The order of a reaction is the sum of the exponents in the rate law equation.
Q: What is the rate constant?
A: The rate constant is a proportionality constant that relates the rate of a reaction to the concentrations of the reactants.
Q: What is the half-life of a reaction?
A: The half-life of a reaction is the time it takes for the concentration of a reactant to decrease by half.
Q: What is the activation energy?
A: The activation energy is the minimum amount of energy required for a reaction to occur.
Q: What is the Arrhenius equation?
A: The Arrhenius equation relates the rate constant of a reaction to the activation energy and temperature.
Q: What is a catalyst?
A: A catalyst is a substance that increases the rate of a reaction by lowering the activation energy.
Q: What is a reaction mechanism?
A: A reaction mechanism is a series of elementary steps that represent the pathway from reactants to products in a chemical reaction.