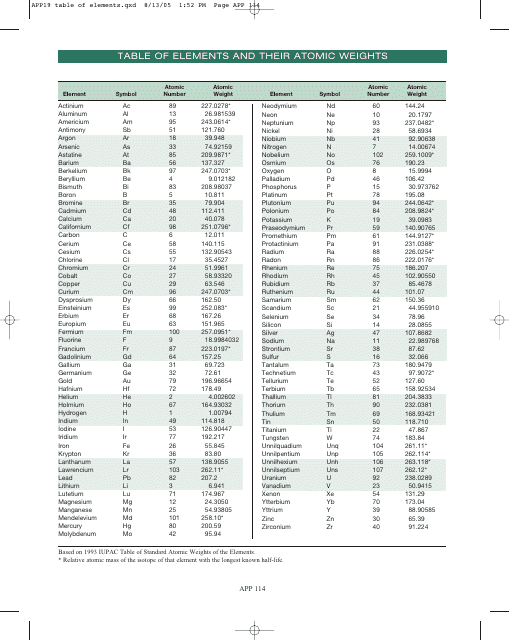
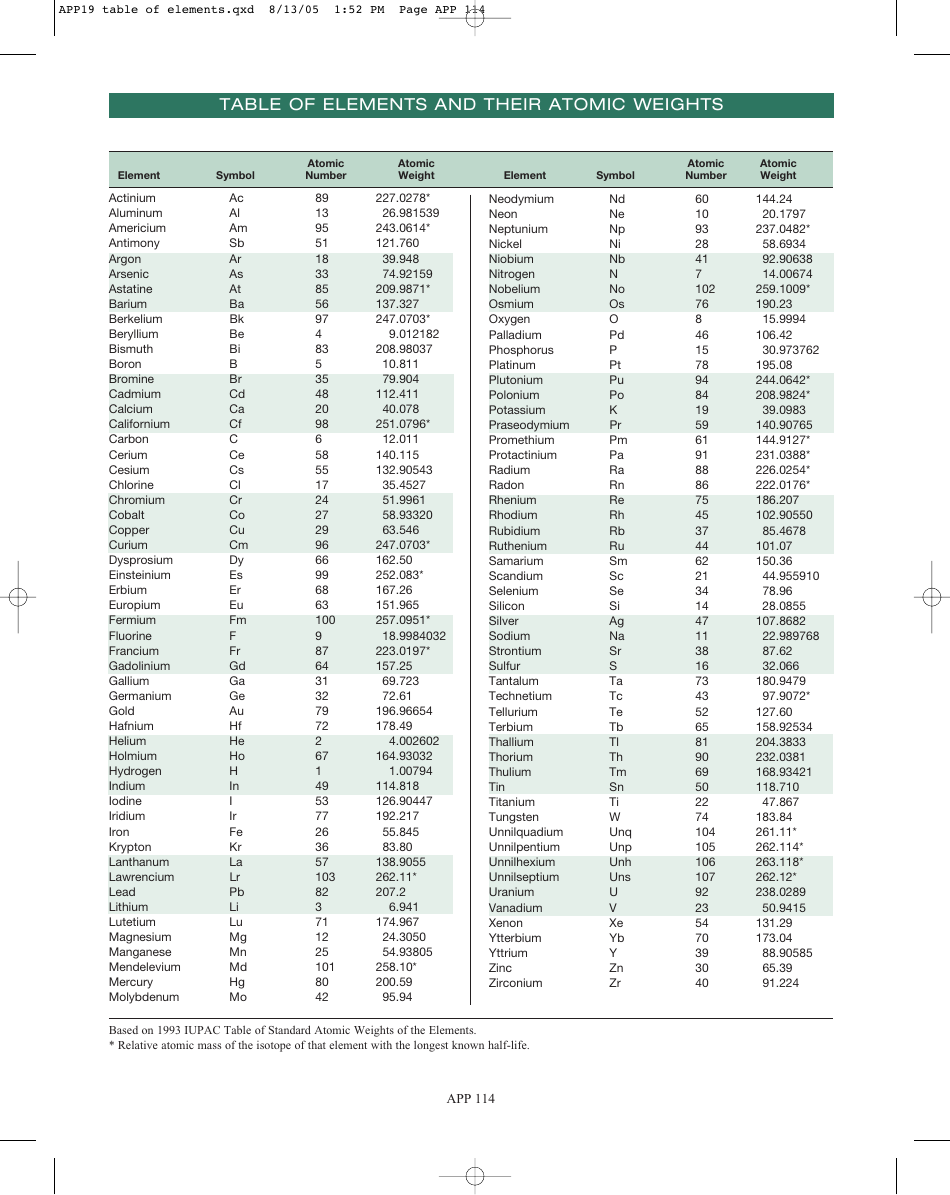
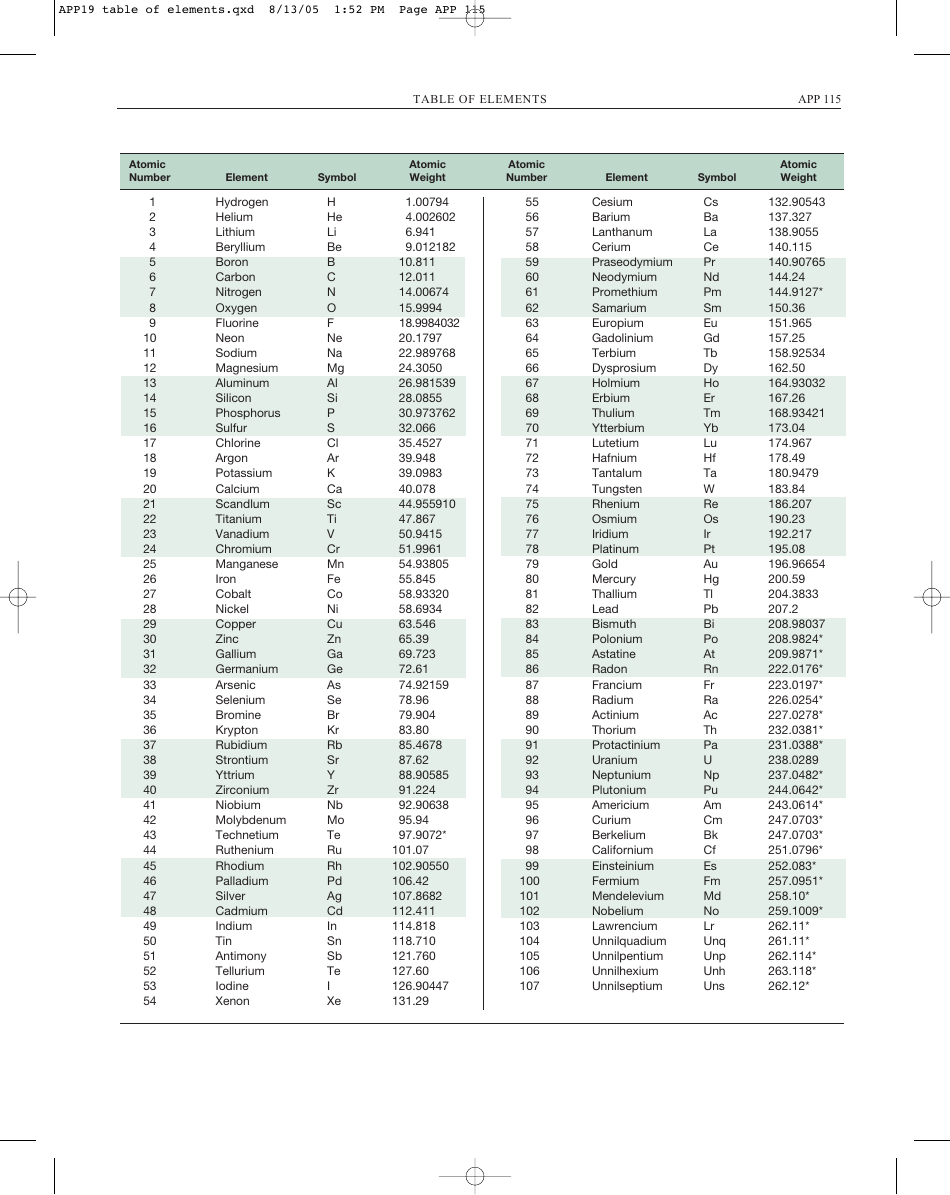
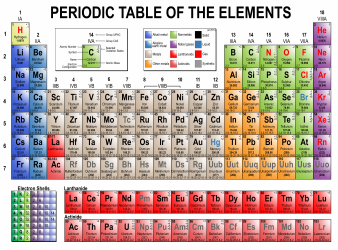
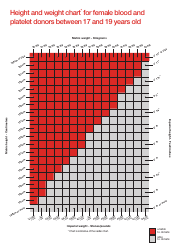
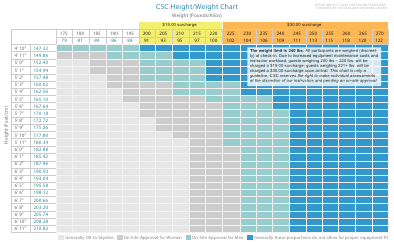
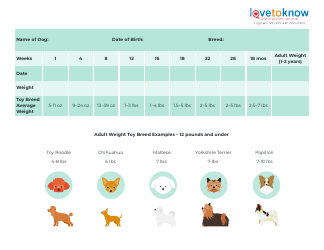
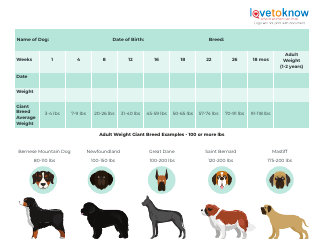
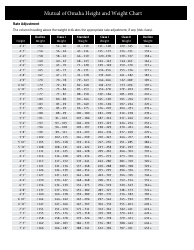
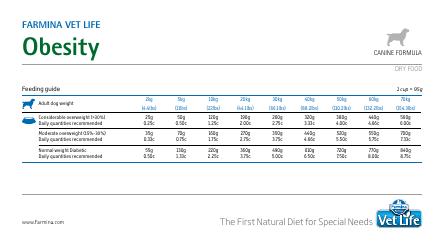
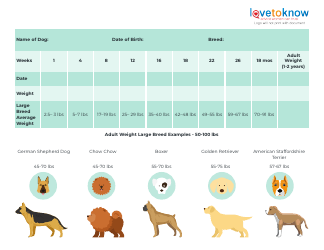
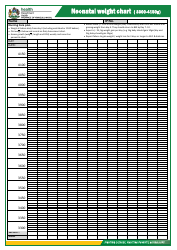
Table of Elements, Atomic Weight Chart
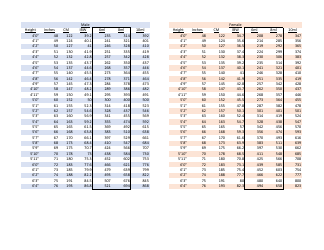
The Table of Elements, also known as the Periodic Table, is a chart that organizes all known chemical elements based on their atomic number, electron configuration, and recurring chemical properties. It provides a systematic way to understand and study the various elements and their relationships to one another. The Atomic Weight Chart, on the other hand, lists the atomic weights of each element, which is the average weight of all the isotopes of an element. It helps scientists and chemists in various fields to calculate and determine the precise measurements and proportions of elements in chemical reactions and scientific experiments.
The Table of Elements, Atomic Weight Chart is maintained and updated by the International Union of Pure and Applied Chemistry (IUPAC).
FAQ
Q: What is the atomic weight of hydrogen?
A: 1.008
Q: What is the atomic weight of carbon?
A: 12.011
Q: What is the atomic weight of oxygen?
A: 15.999
Q: What is the atomic weight of gold?
A: 196.967
Q: What is the atomic weight of uranium?
A: 238.028
Q: What is the atomic weight of lead?
A: 207.2