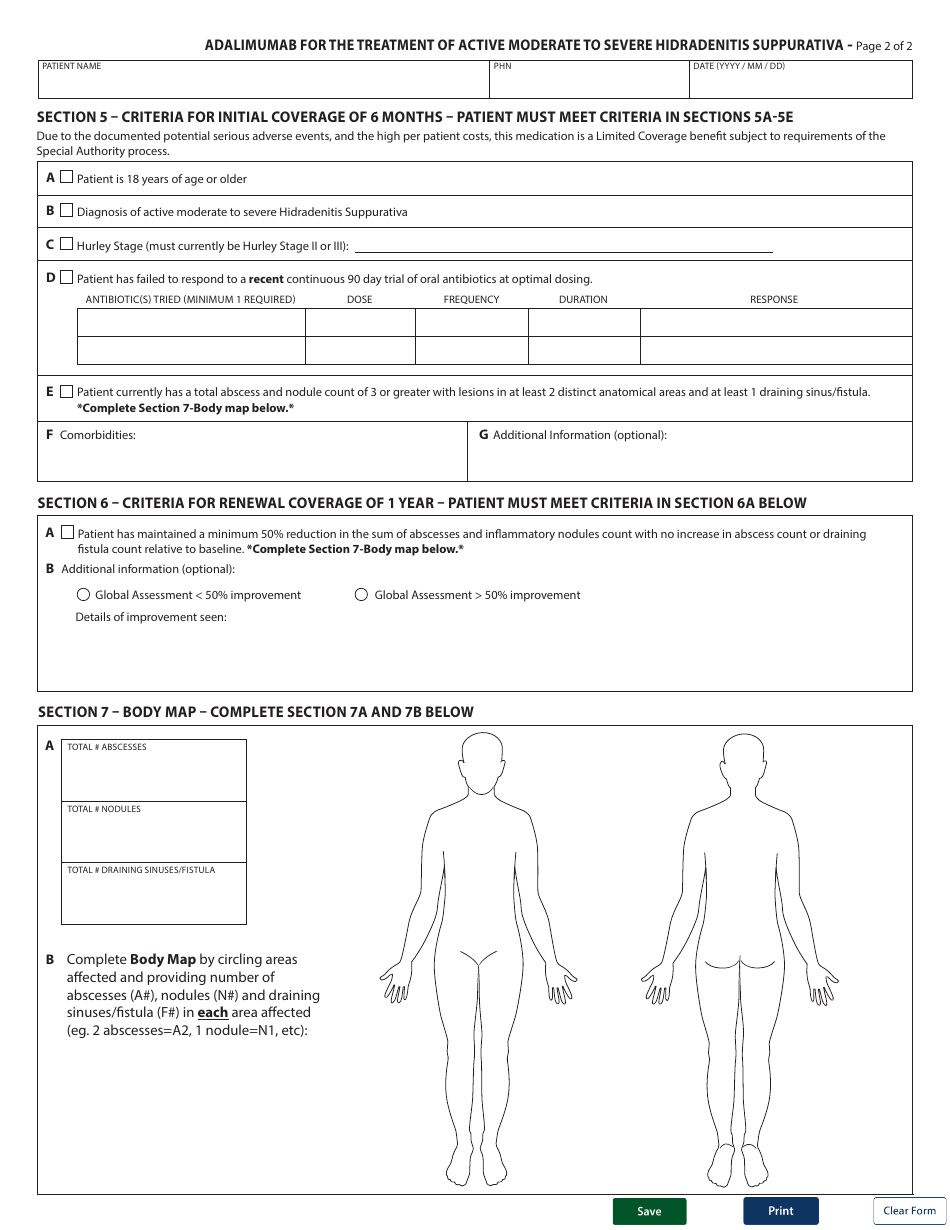
Form HLTH5485 Special Authority Request - Adalimumab for the Treatment of Active Moderate to Severe Hidradenitis Suppurativa - British Columbia, Canada
Form HLTH5485 Special Authority Request - Adalimumab for the Treatment of Active Moderate to Severe Hidradenitis Suppurativa - British Columbia, Canada is used to request special authority for the use of the medication Adalimumab in the treatment of active moderate to severe Hidradenitis Suppurativa in the province of British Columbia, Canada.
The form HLTH5485 Special Authority Request - Adalimumab for the Treatment of Active Moderate to Severe Hidradenitis Suppurativa in British Columbia, Canada is typically filed by the individual seeking the treatment.
FAQ
Q: What is HLTH5485?
A: HLTH5485 is a Special Authority Request form for the treatment of active moderate to severe Hidradenitis Suppurativa with Adalimumab in British Columbia, Canada.
Q: What is Hidradenitis Suppurativa?
A: Hidradenitis Suppurativa is a chronic skin condition characterized by painful and recurrent abscesses or nodules in areas of the body with apocrine glands, such as the armpits and groin.
Q: What is Adalimumab?
A: Adalimumab is a medication commonly used to treat autoimmune conditions such as rheumatoid arthritis and psoriasis. It has also been found to be effective in the treatment of Hidradenitis Suppurativa.
Q: What is a Special Authority Request?
A: A Special Authority Request is a process by which certain medications are approved for coverage by the provincial health care program in British Columbia, Canada.
Q: How do I fill out the HLTH5485 form?
A: The HLTH5485 form should be completed by your healthcare provider, who will provide the necessary information about your diagnosis and treatment history.
Q: Who is eligible for Adalimumab treatment for Hidradenitis Suppurativa?
A: Eligibility for Adalimumab treatment for Hidradenitis Suppurativa is determined by the specific criteria outlined in the Special Authority Request form.
Q: How long does it take for a Special Authority Request to be reviewed?
A: The review process for a Special Authority Request can vary, but typically takes about 4-6 weeks.
Q: What happens after the Special Authority Request is approved?
A: After the Special Authority Request is approved, you will be able to access Adalimumab for the treatment of active moderate to severe Hidradenitis Suppurativa through the provincial health care program in British Columbia, Canada.
Q: Are there any side effects of Adalimumab?
A: Like any medication, Adalimumab can have side effects. These can include injection site reactions, infections, and an increased risk of certain types of cancer. It is important to discuss potential side effects with your healthcare provider.