Drug Master File (Dmf) Application Form - Canada
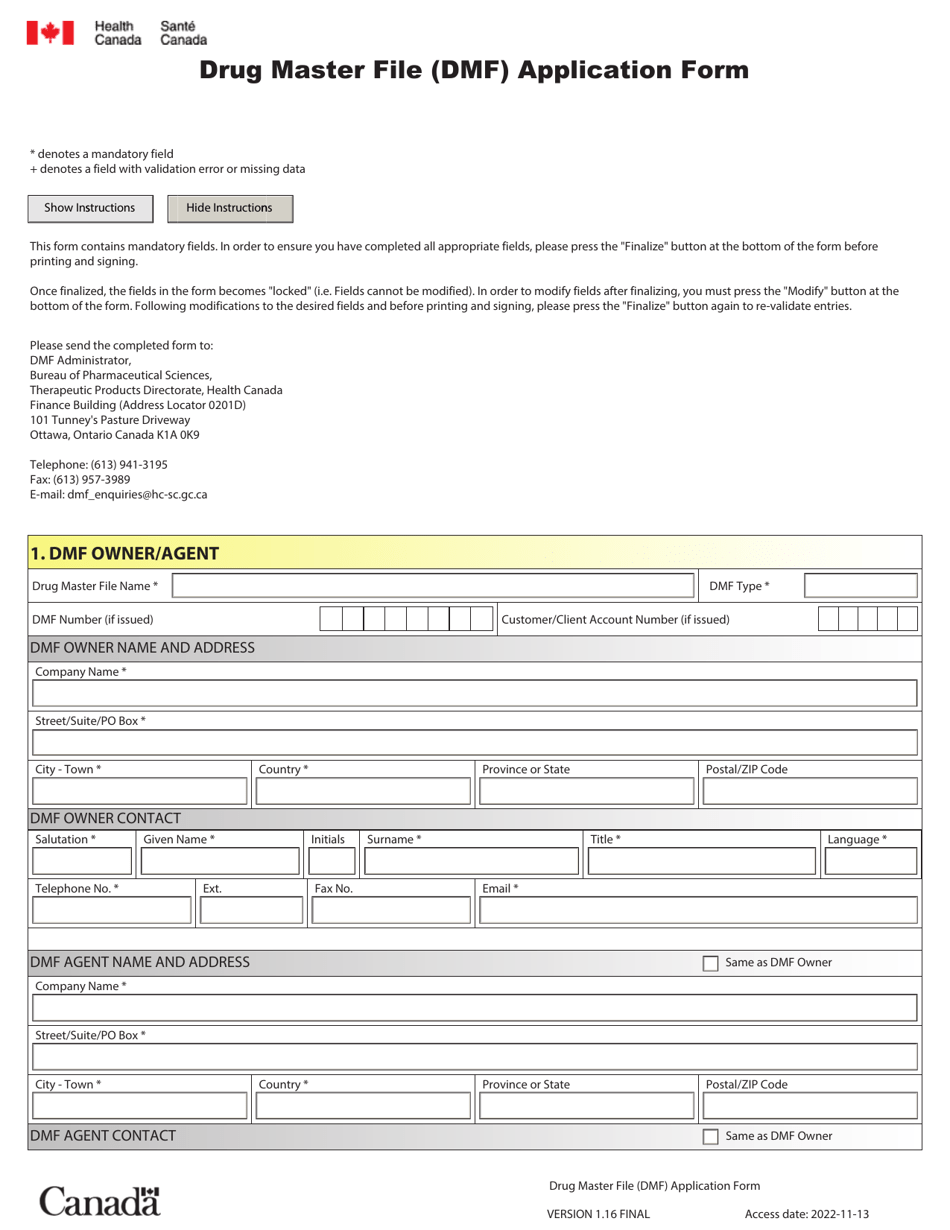
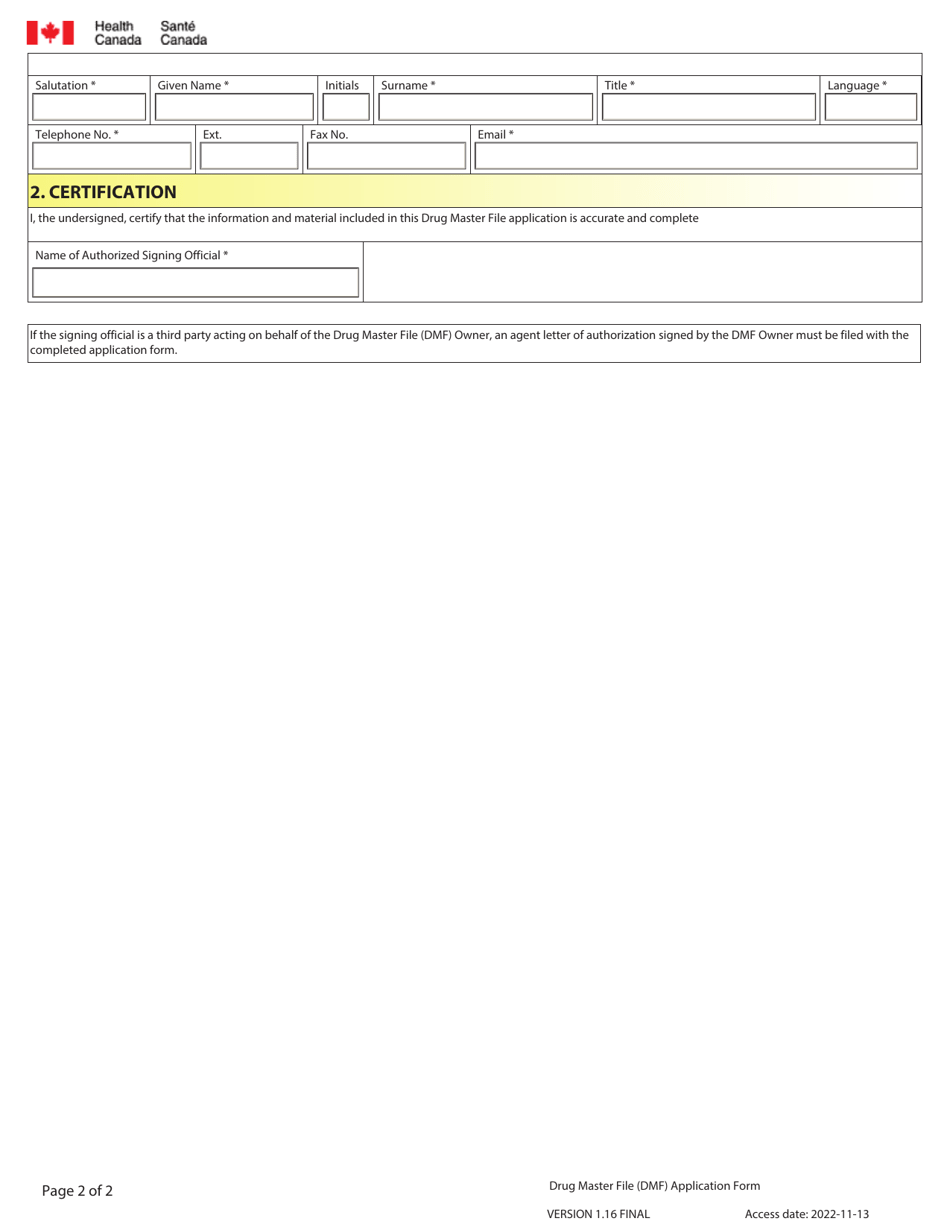
The Drug Master File (DMF) application form in Canada is used for the submission of information about a drug product to Health Canada. It is a confidential document that contains detailed information about the quality, safety, and efficacy of the drug. The purpose of the DMF is to support the regulatory review process and provide information to Health Canada for drug evaluation and approval.
In Canada, the Drug Master File (DMF) application form is filed by the pharmaceutical company or the manufacturer.
FAQ
Q: What is a Drug Master File (DMF) application?
A: A DMF application is a submission made to Health Canada to provide detailed information about the manufacturing, processing, and formulation of a drug.
Q: What is the purpose of a DMF?
A: The purpose of a DMF is to allow a drug manufacturer to provide confidential and proprietary information to Health Canada without disclosing it to the public.
Q: Who can submit a DMF application?
A: Any drug manufacturer or other interested party can submit a DMF application.
Q: What information should be included in a DMF application?
A: A DMF application should include detailed information on the drug's composition, manufacturing process, quality control procedures, and any other relevant information.
Q: How long does it take for Health Canada to review a DMF application?
A: The review process for a DMF application typically takes between 90 to 180 days, depending on the complexity of the application.
Q: What happens after Health Canada approves a DMF application?
A: After approval, the DMF is assigned a DMF number and can be referenced by drug manufacturers in their own drug applications.
Q: Can the information in a DMF be accessed by the public?
A: No, the information in a DMF is considered confidential and is not accessible to the public.
Q: Is a DMF application required for every drug manufactured in Canada?
A: No, a DMF application is not required for every drug. It is only required for drugs where the manufacturer wants to provide confidential information to Health Canada.
Q: Is there a fee for submitting a DMF application?
A: Yes, there is a fee associated with submitting a DMF application. The fee depends on the type of DMF and the annual sales of the drug.