This version of the form is not currently in use and is provided for reference only. Download this version of
Form CMS-116
for the current year.
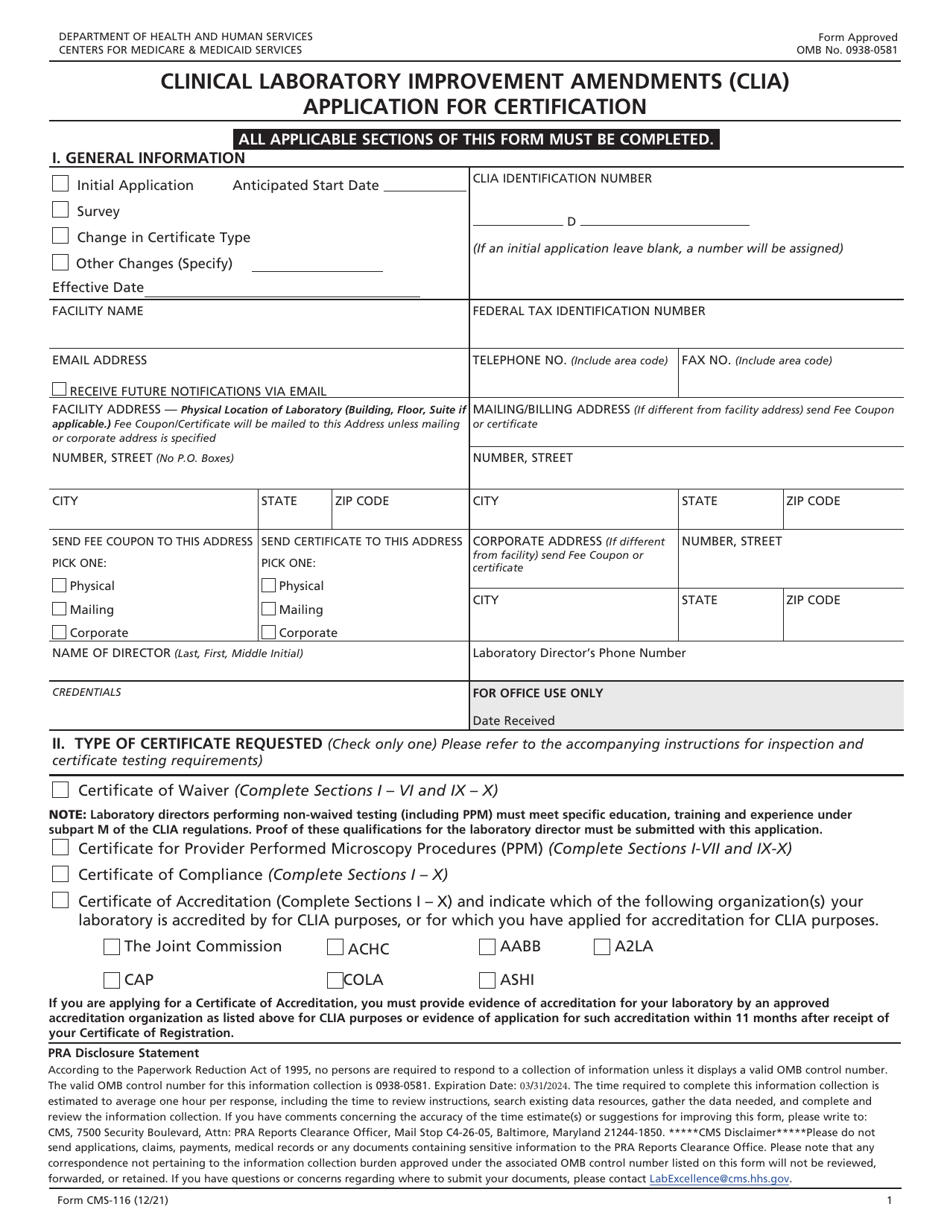
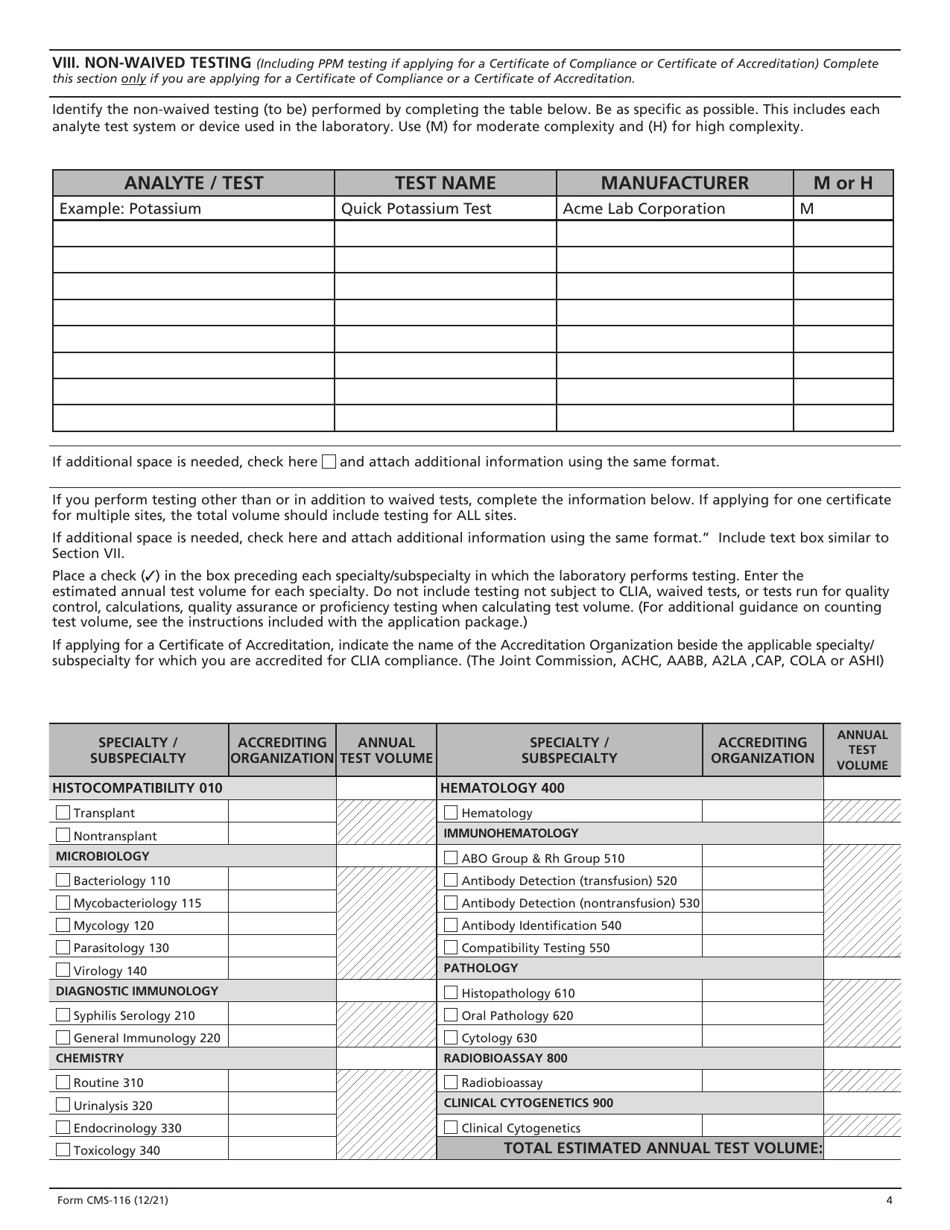
Form CMS-116 Clinical Laboratory Improvement Amendments (Clia) Application for Certification
What Is Form CMS-116?
This is a legal form that was released by the U.S. Department of Health and Human Services - Centers for Medicare and Medicaid Services on December 1, 2021 and used country-wide. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is Form CMS-116?
A: Form CMS-116 is the Clinical Laboratory Improvement Amendments (CLIA) Application for Certification.
Q: What is the purpose of Form CMS-116?
A: The purpose of Form CMS-116 is to apply for certification under the CLIA program for clinical laboratories.
Q: Who needs to fill out Form CMS-116?
A: Clinical laboratories that perform laboratory testing on human specimens for the purpose of diagnosis, prevention, or treatment of disease or impairment in the United States are required to fill out Form CMS-116.
Q: Are there any fees associated with submitting Form CMS-116?
A: Yes, there are fees associated with submitting Form CMS-116. The fee amount depends on the type of laboratory and the tests performed.
Q: How long does it take to process Form CMS-116?
A: The processing time for Form CMS-116 can vary, but it typically takes around 60 days from the date of receipt for the application to be processed.
Q: What happens after Form CMS-116 is processed?
A: After Form CMS-116 is processed, the laboratory will receive a CLIA certificate if it meets the requirements for certification.
Q: What is the CLIA program?
A: The CLIA program is a federal certification program that regulates and ensures the quality of laboratory testing performed on human specimens for the purpose of diagnosis, prevention, or treatment of disease or impairment.
Q: What are the benefits of CLIA certification?
A: CLIA certification ensures that laboratory testing is performed accurately and reliably, which promotes patient safety and quality healthcare.
Form Details:
- Released on December 1, 2021;
- The latest available edition released by the U.S. Department of Health and Human Services - Centers for Medicare and Medicaid Services;
- Easy to use and ready to print;
- Yours to fill out and keep for your records;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form CMS-116 by clicking the link below or browse more documents and templates provided by the U.S. Department of Health and Human Services - Centers for Medicare and Medicaid Services.