This version of the form is not currently in use and is provided for reference only. Download this version of
the document
for the current year.
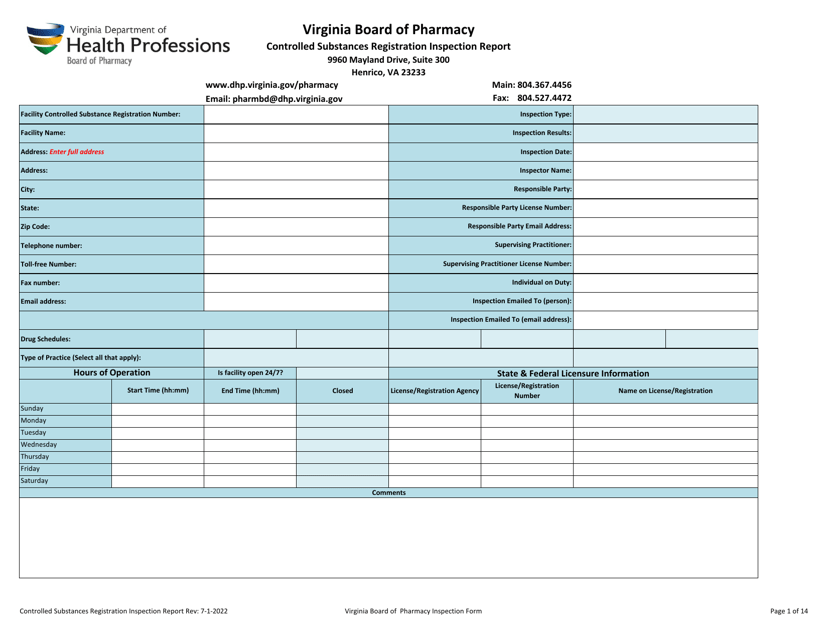
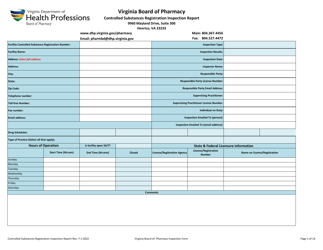
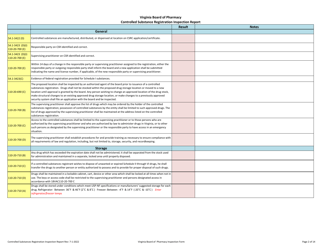
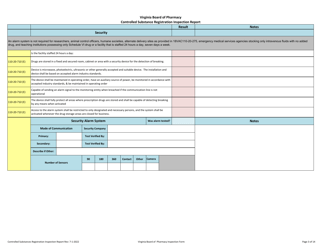
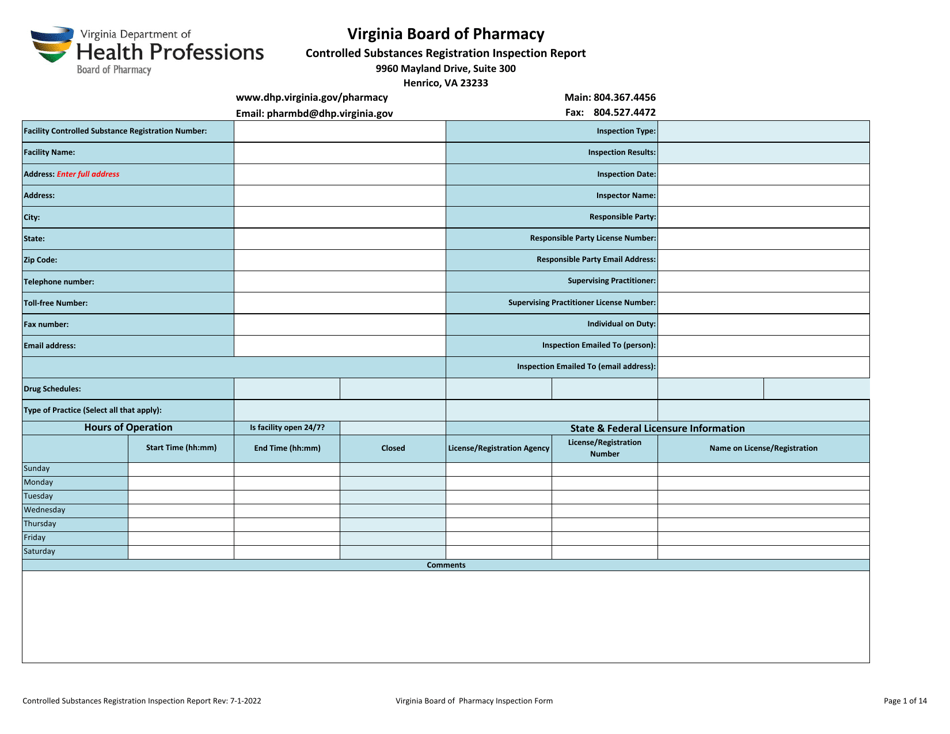
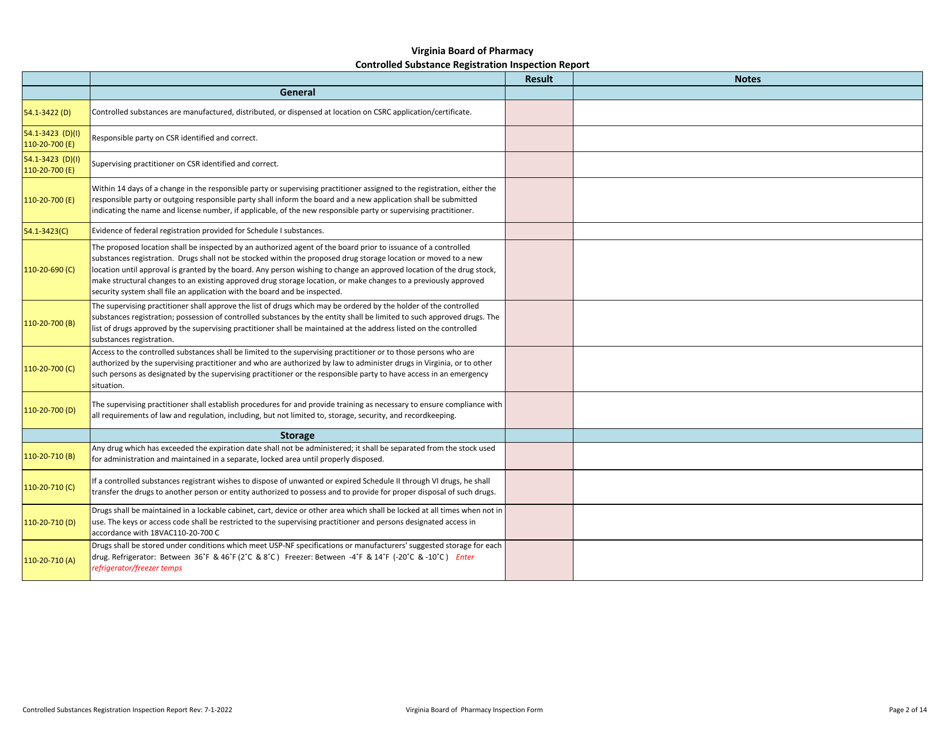
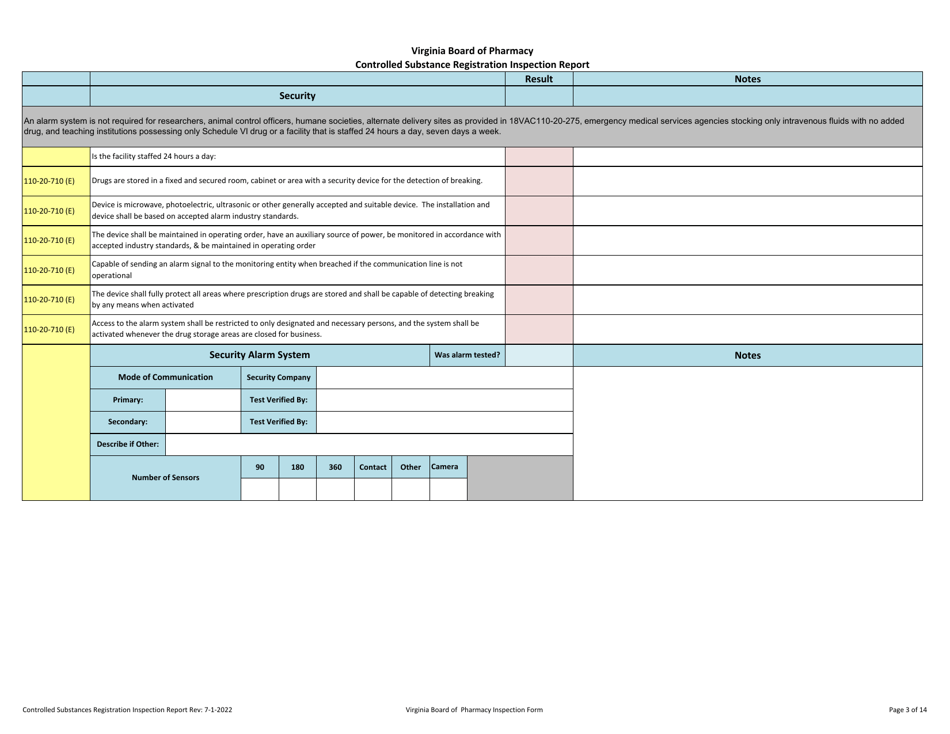
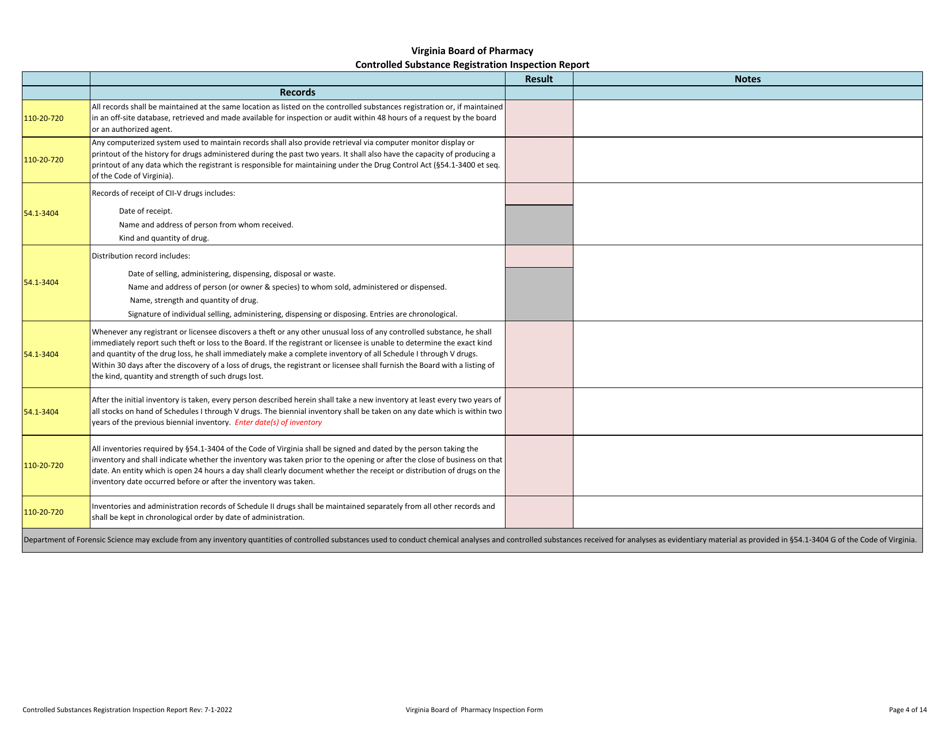
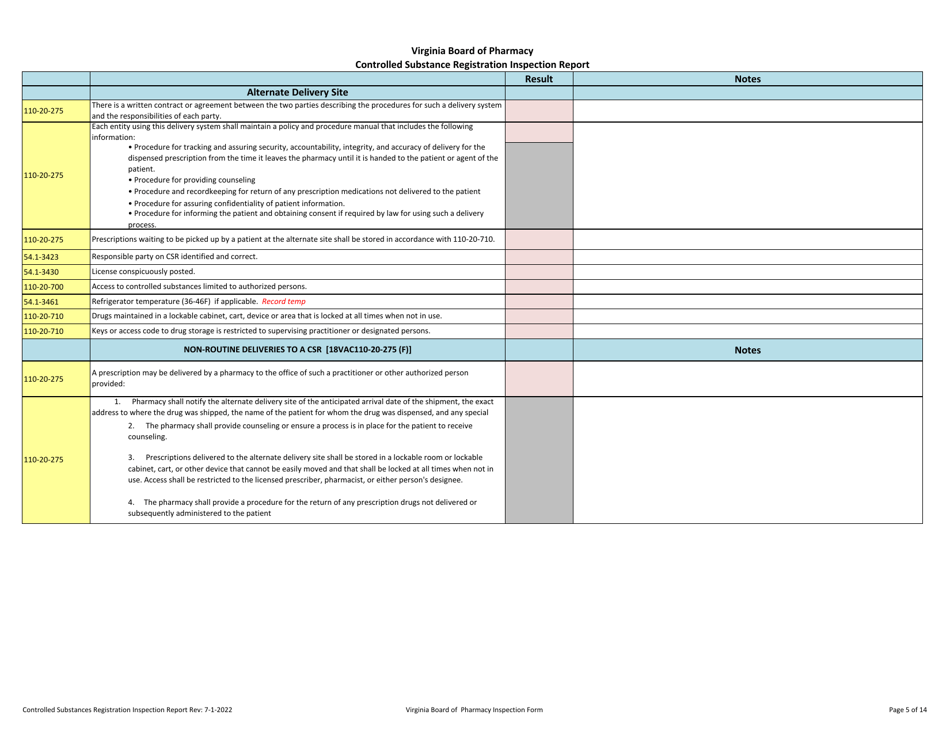
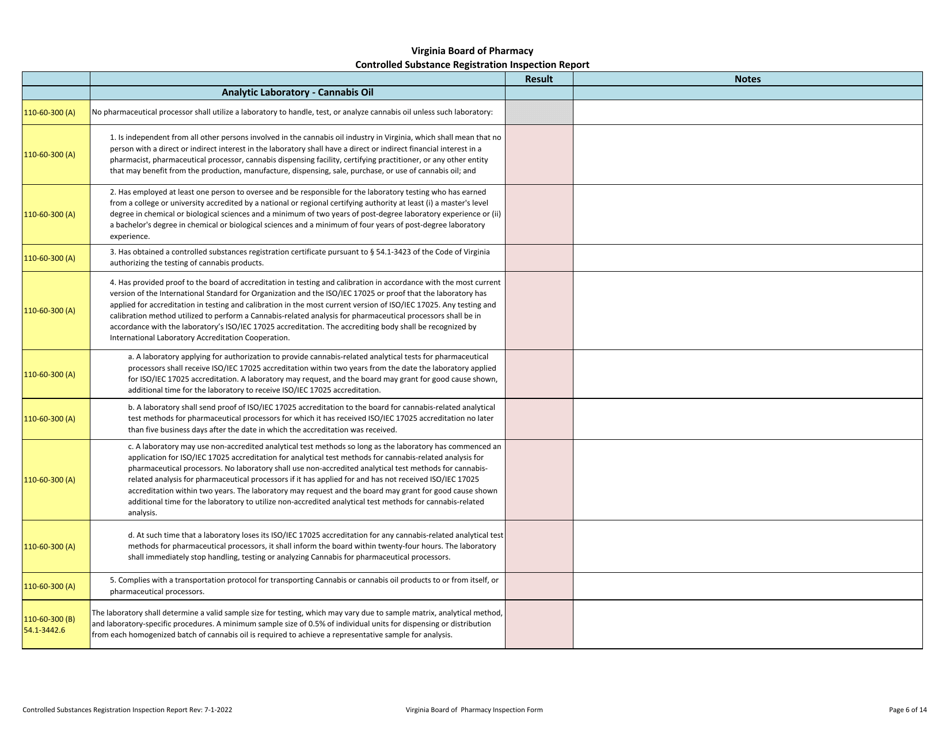
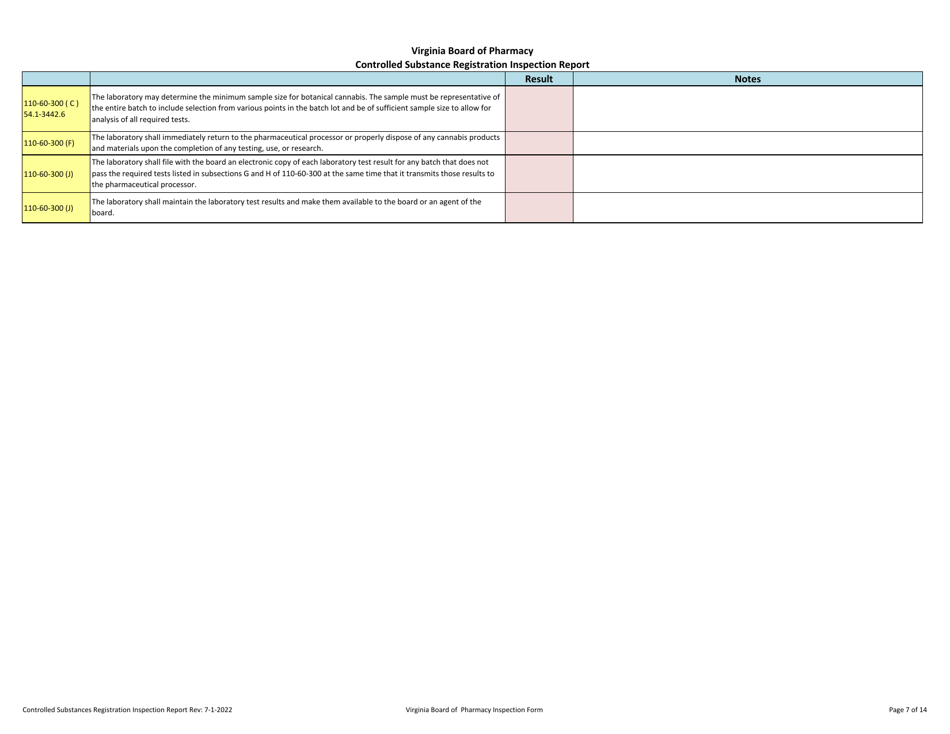
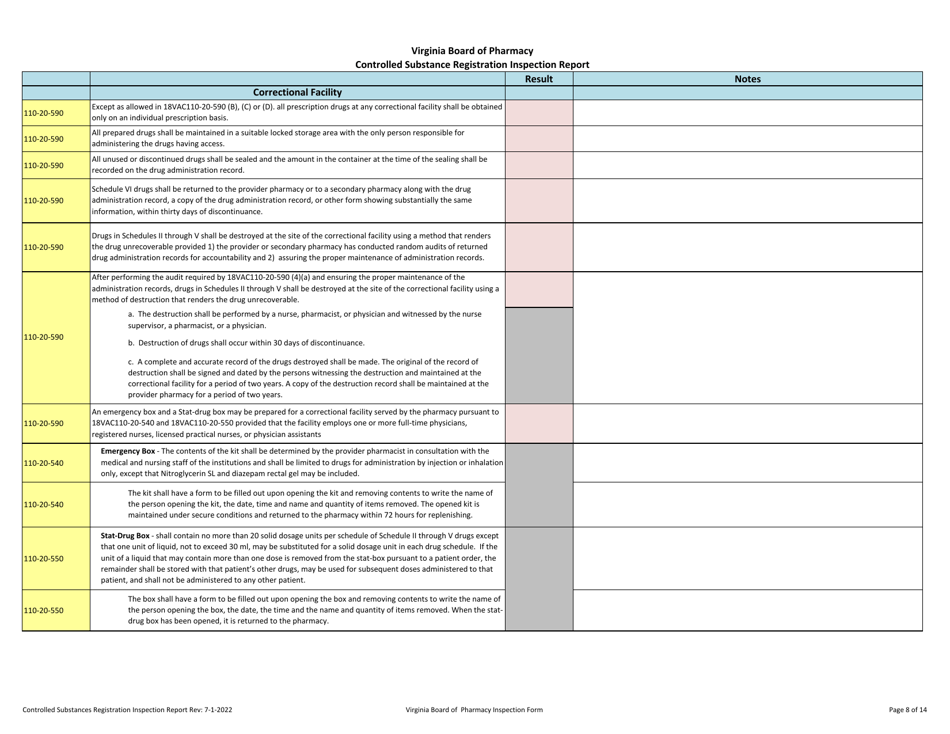
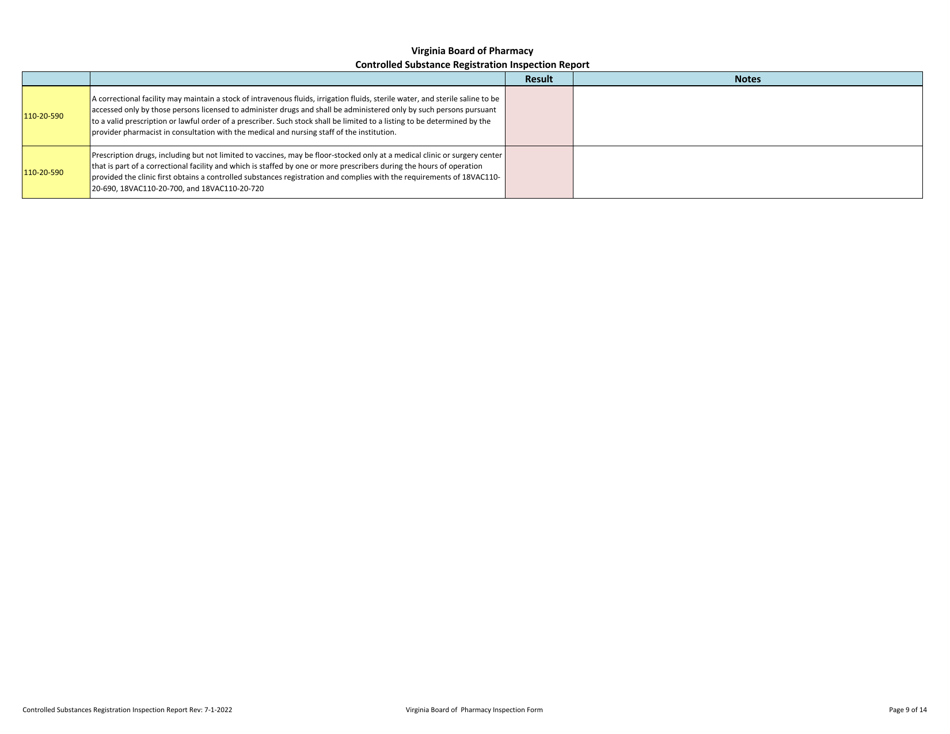
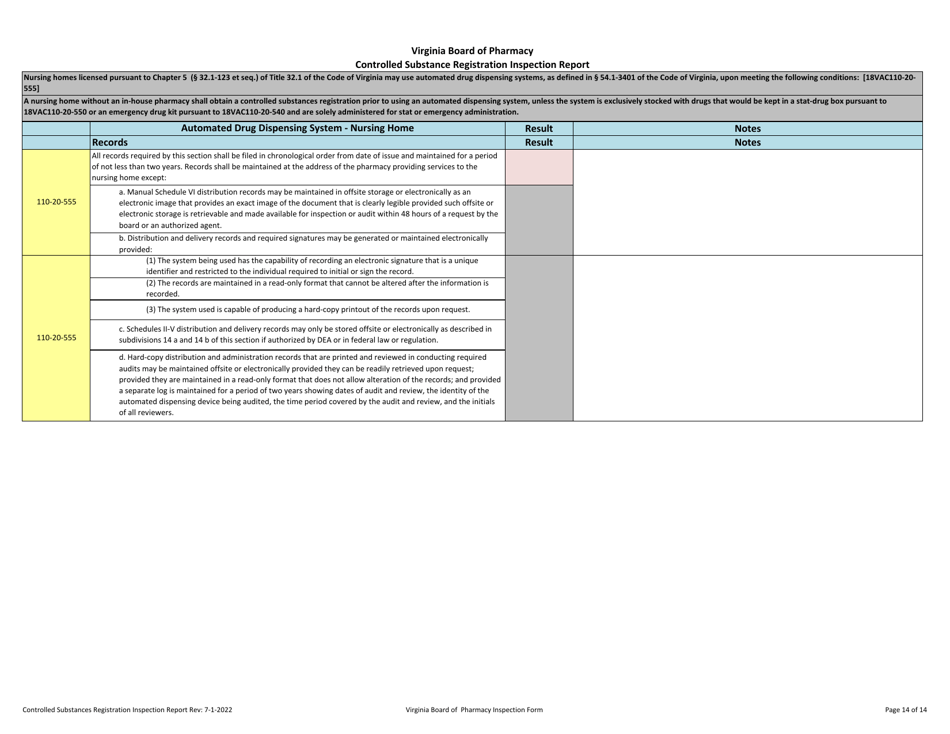
Controlled Substances Registration Inspection Report - Virginia
Controlled Substances Registration Inspection Report is a legal document that was released by the Virginia Department of Health Professions - a government authority operating within Virginia.
FAQ
Q: What is a Controlled Substances Registration Inspection Report?
A: A Controlled Substances Registration Inspection Report is a document that provides information about an inspection conducted on an entity's controlled substances registration in Virginia.
Q: What does a Controlled Substances Registration Inspection Report contain?
A: A Controlled Substances Registration Inspection Report typically contains details about the entity being inspected, the inspection process, any violations or observations made during the inspection, and recommendations for corrective actions.
Q: Who conducts Controlled Substances Registration inspections in Virginia?
A: Controlled Substances Registration inspections in Virginia are typically conducted by the Virginia Department of Health Professions, specifically the Board of Pharmacy.
Q: What is the purpose of a Controlled Substances Registration inspection?
A: The purpose of a Controlled Substances Registration inspection is to ensure compliance with laws and regulations related to the handling, storage, and dispensing of controlled substances in Virginia.
Q: What should an entity do if violations or observations are found during a Controlled Substances Registration inspection?
A: If violations or observations are found during a Controlled Substances Registration inspection, the entity should take necessary corrective actions to address these issues and prevent their recurrence.
Form Details:
- Released on July 1, 2022;
- The latest edition currently provided by the Virginia Department of Health Professions;
- Ready to use and print;
- Easy to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a printable version of the form by clicking the link below or browse more documents and templates provided by the Virginia Department of Health Professions.