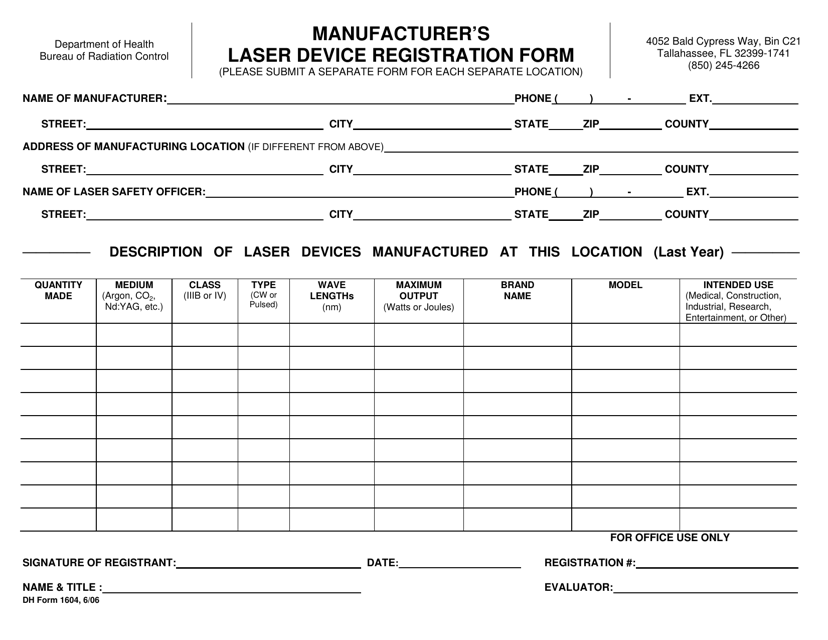
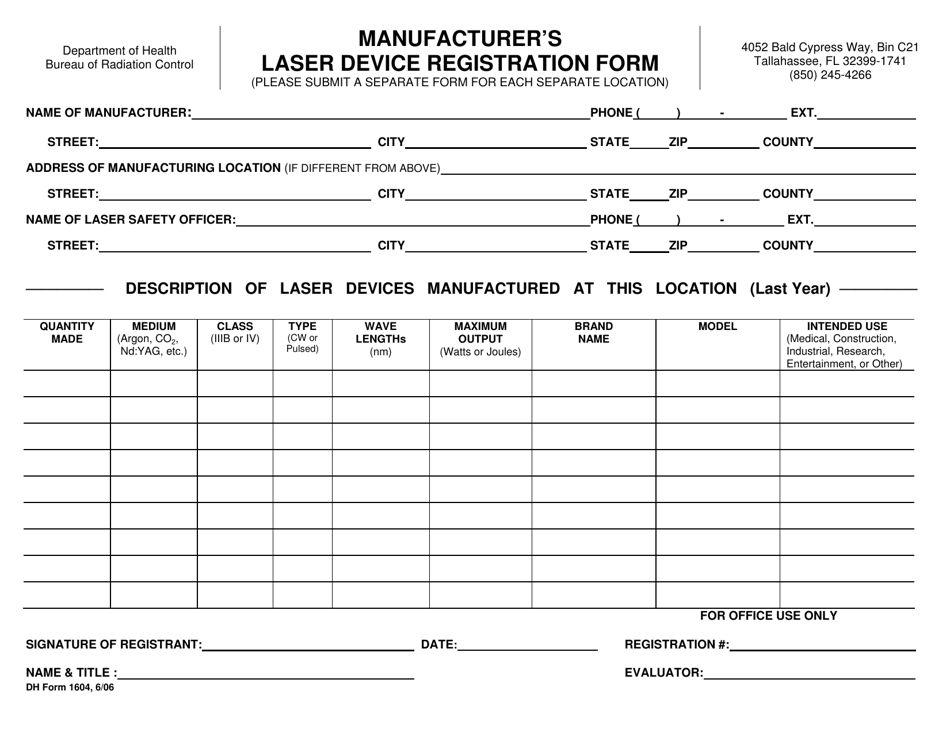
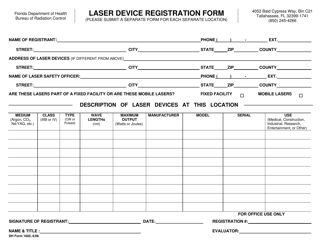
DH Form 1604 Manufacturer's Laser Device Registration Form - Florida
What Is DH Form 1604?
This is a legal form that was released by the Florida Department of Health - a government authority operating within Florida. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is DH Form 1604?
A: DH Form 1604 is the Manufacturer's Laser Device Registration Form.
Q: What is the purpose of DH Form 1604?
A: The purpose of DH Form 1604 is to register laser devices manufactured in Florida.
Q: Who needs to fill out DH Form 1604?
A: Manufacturers of laser devices in Florida need to fill out DH Form 1604.
Q: Is DH Form 1604 specific to Florida?
A: Yes, DH Form 1604 is specific to manufacturers in Florida.
Q: Are there any fees associated with DH Form 1604?
A: There may be fees associated with DH Form 1604, depending on the requirements set by the Florida government agency.
Q: What other documents may be required with DH Form 1604?
A: Additional documentation, such as product specifications and safety information, may be required with DH Form 1604.
Q: What happens after submitting DH Form 1604?
A: After submitting DH Form 1604, you may receive a certificate of registration for your laser device.
Form Details:
- Released on June 1, 2006;
- The latest edition provided by the Florida Department of Health;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a printable version of DH Form 1604 by clicking the link below or browse more documents and templates provided by the Florida Department of Health.