Preparation of Salts Chemistry Worksheet
The Preparation of Salts Chemistry Worksheet is a learning tool designed to help students understand and practice the process of preparing salts. It contains exercises and questions related to the methods, equations, and concepts involved in salt preparation.
FAQ
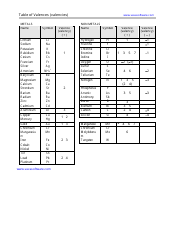
Q: What is a salt?
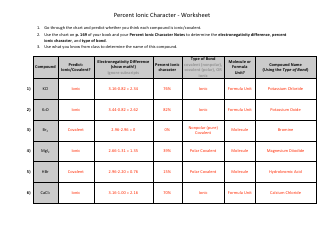
A: A salt is a compound formed when an acid reacts with a base.
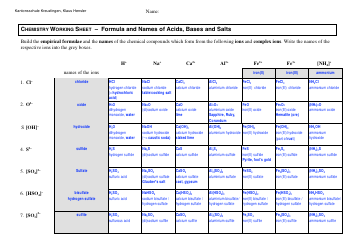
Q: How are salts prepared?
A: Salts can be prepared by the neutralization reaction between an acid and a base.
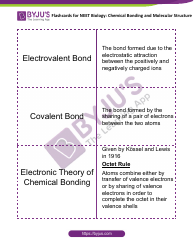
Q: What is the neutralization reaction?
A: The neutralization reaction is a chemical reaction between an acid and a base, resulting in the formation of a salt and water.
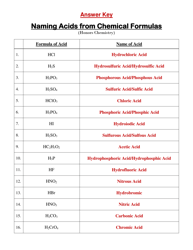
Q: Give an example of a salt preparation reaction.
A: One example is the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) to form sodium chloride (NaCl) and water (H2O).
Q: What are some common methods for preparing salts?
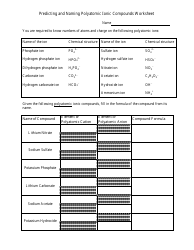
A: Some common methods for preparing salts include reacting an acid with a metal, reacting an acid with a metal oxide, and reacting an acid with a metal carbonate.
Q: Why are salts important?
A: Salts have various uses, such as food seasoning, medicine production, and industrial applications.