Formal Charge and Resonance Chemistry Worksheet
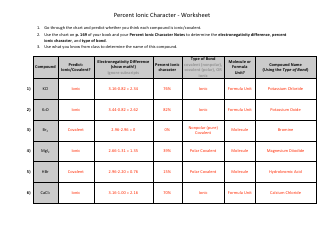
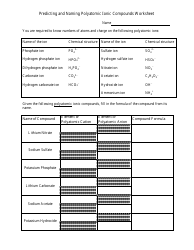
A Formal Charge and Resonance Chemistry Worksheet is typically used as a learning tool in chemistry education. It helps students understand and practice the concept of formal charge and resonance in chemical compounds. The worksheet may include questions or problems that require students to calculate formal charges of atoms in molecules and understand the concept of resonance structures.
The formal charge and resonance chemistry worksheet is typically filed by the teacher or instructor who assigns the worksheet to their students. The purpose of this worksheet is to help students practice and understand concepts related to formal charge and resonance in chemistry.
FAQ
Q: What is formal charge?
A: Formal charge is the charge assigned to an atom in a Lewis structure.
Q: How is formal charge calculated?
A: Formal charge is calculated by subtracting the sum of non-bonded electrons and half of the shared electrons from the number of valence electrons.
Q: Why is formal charge important in chemistry?
A: Formal charge helps determine the most stable Lewis structure and understand the distribution of charges in a molecule.
Q: What is resonance?
A: Resonance is when multiple Lewis structures can be drawn for a molecule, indicating that the true structure is a combination of these resonance structures.
Q: How is resonance indicated in a Lewis structure?
A: Resonance is indicated by using double-headed arrows connecting the different resonance structures.
Q: What is the purpose of resonance?
A: Resonance helps explain the stability and reactivity of molecules, especially those with delocalized electrons.