Form CDC50.153 (E) Babesiosis Case Report Form
What Is Form CDC50.153 (E)?
This is a legal form that was released by the U.S. Department of Health and Human Services - Centers for Disease Control and Prevention on November 1, 2016 and used country-wide. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is Form CDC50.153 (E)?
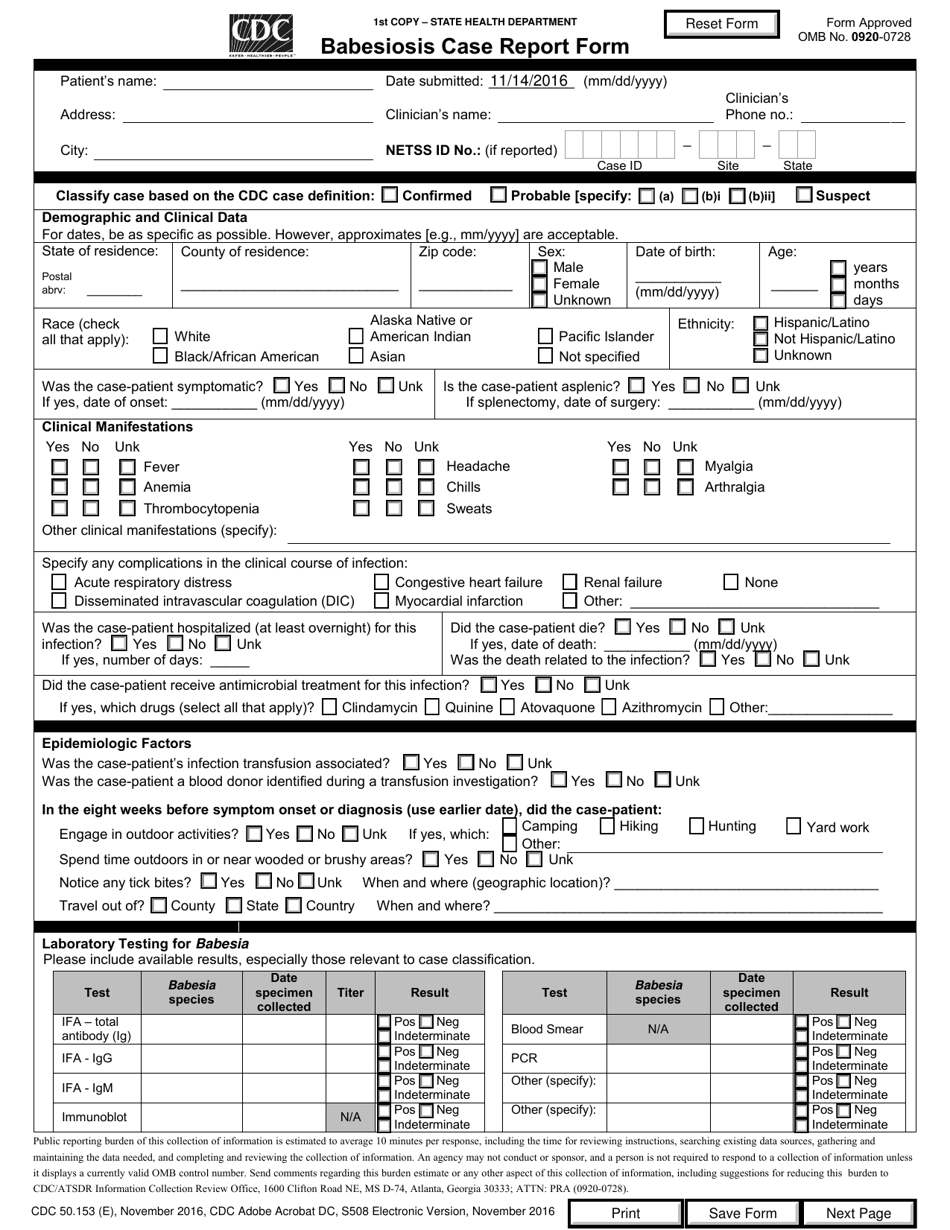
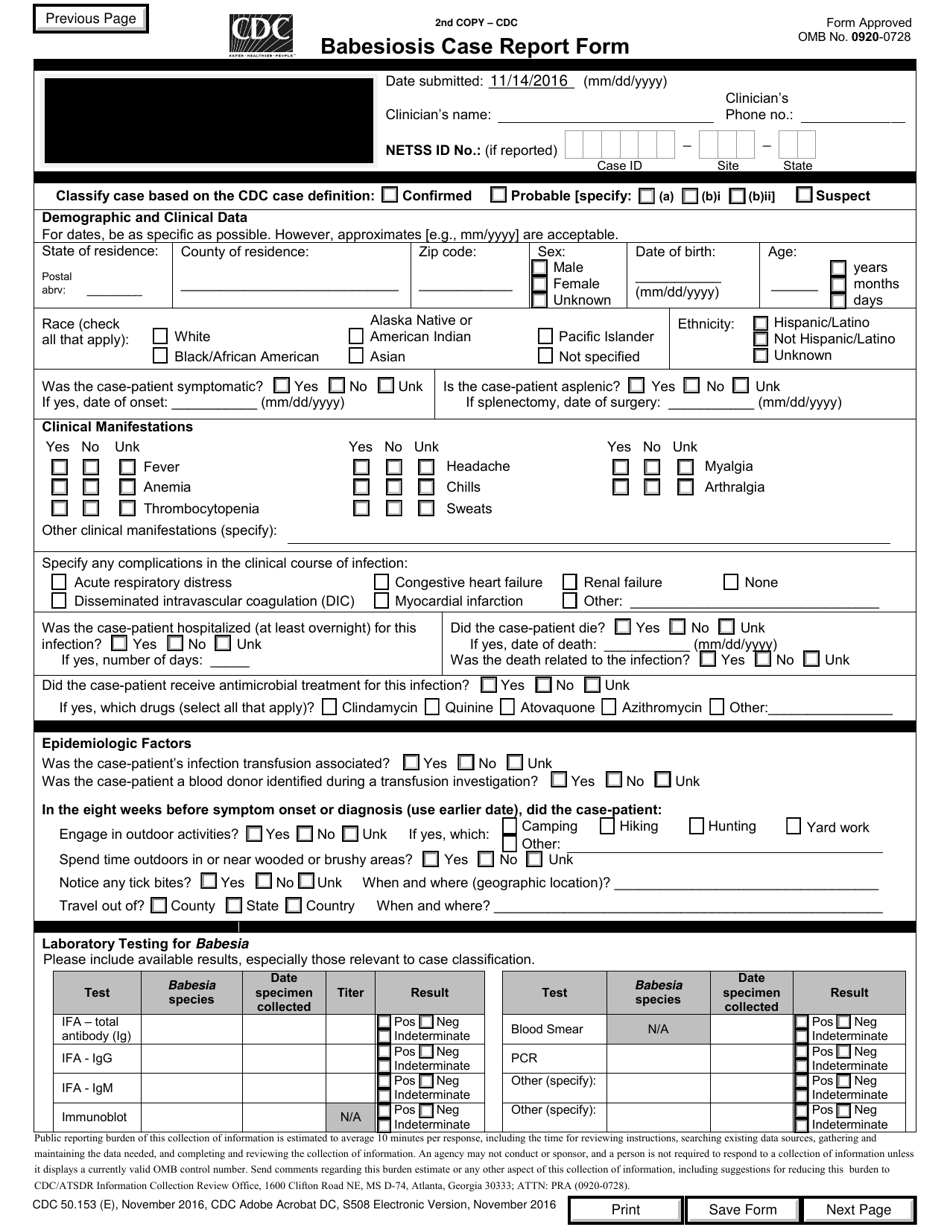
A: Form CDC50.153 (E) is the Babesiosis Case Report Form.
Q: What is the purpose of Form CDC50.153 (E)?
A: The purpose of Form CDC50.153 (E) is to report cases of Babesiosis.
Q: Who should use Form CDC50.153 (E)?
A: Form CDC50.153 (E) should be used by healthcare providers or laboratories to report cases of Babesiosis.
Q: What information is required on Form CDC50.153 (E)?
A: Form CDC50.153 (E) requires information such as patient details, signs and symptoms, laboratory test results, and treatment information.
Q: Is Form CDC50.153 (E) mandatory?
A: The use of Form CDC50.153 (E) may be mandatory in certain jurisdictions, so it is important to check with local health authorities.
Q: Is Form CDC50.153 (E) confidential?
A: Yes, Form CDC50.153 (E) contains confidential patient information and should be handled in accordance with privacy regulations.
Q: Is there a deadline for submitting Form CDC50.153 (E)?
A: The deadline for submitting Form CDC50.153 (E) may vary depending on local reporting requirements, so it is important to check with local health authorities.
Form Details:
- Released on November 1, 2016;
- The latest available edition released by the U.S. Department of Health and Human Services - Centers for Disease Control and Prevention;
- Easy to use and ready to print;
- Yours to fill out and keep for your records;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form CDC50.153 (E) by clicking the link below or browse more documents and templates provided by the U.S. Department of Health and Human Services - Centers for Disease Control and Prevention.