Application for Registration as a Non-resident Medical Equipment Supplier - Virginia
Application for Registration as a Non-resident Medical Equipment Supplier is a legal document that was released by the Virginia Department of Health Professions - a government authority operating within Virginia.
FAQ
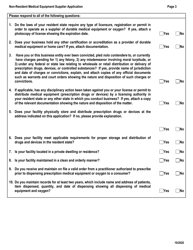
Q: Who can apply for registration as a non-resident medical equipment supplier in Virginia?
A: Any medical equipment supplier that is not based in Virginia can apply.
Q: What is the purpose of this registration?
A: The purpose of the registration is to allow non-resident medical equipment suppliers to conduct business in Virginia.
Q: What is the process for applying for registration?
A: You need to complete an application form and submit it along with the required fee.
Q: Is there a fee for the registration?
A: Yes, there is a fee for the registration. The amount of the fee may vary.
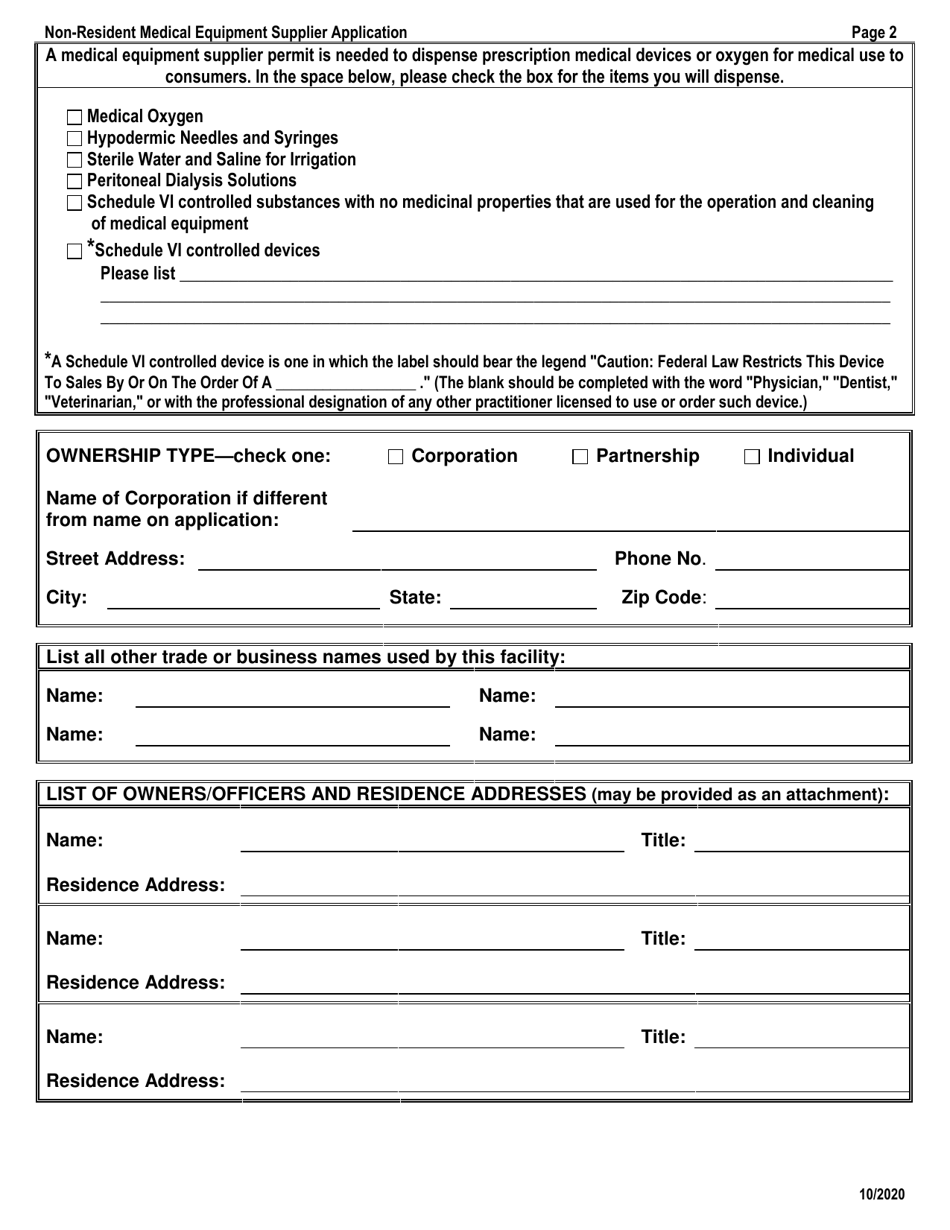
Q: Are there any additional requirements for registration?
A: Yes, you may be required to provide proof of liability insurance and submit to an inspection.
Q: How long does the registration process take?
A: The processing time for the registration application can vary, but it typically takes several weeks.
Q: Once registered, is there anything else I need to do?
A: You will be required to renew your registration annually and notify the Virginia Department of Health of any changes to your business information.
Q: What happens if I fail to register?
A: If you fail to register as a non-resident medical equipment supplier in Virginia, you may be subject to penalties and restrictions on doing business in the state.
Form Details:
- Released on October 1, 2020;
- The latest edition currently provided by the Virginia Department of Health Professions;
- Ready to use and print;
- Easy to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a printable version of the form by clicking the link below or browse more documents and templates provided by the Virginia Department of Health Professions.