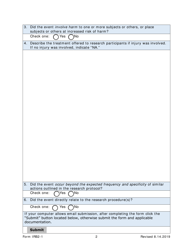
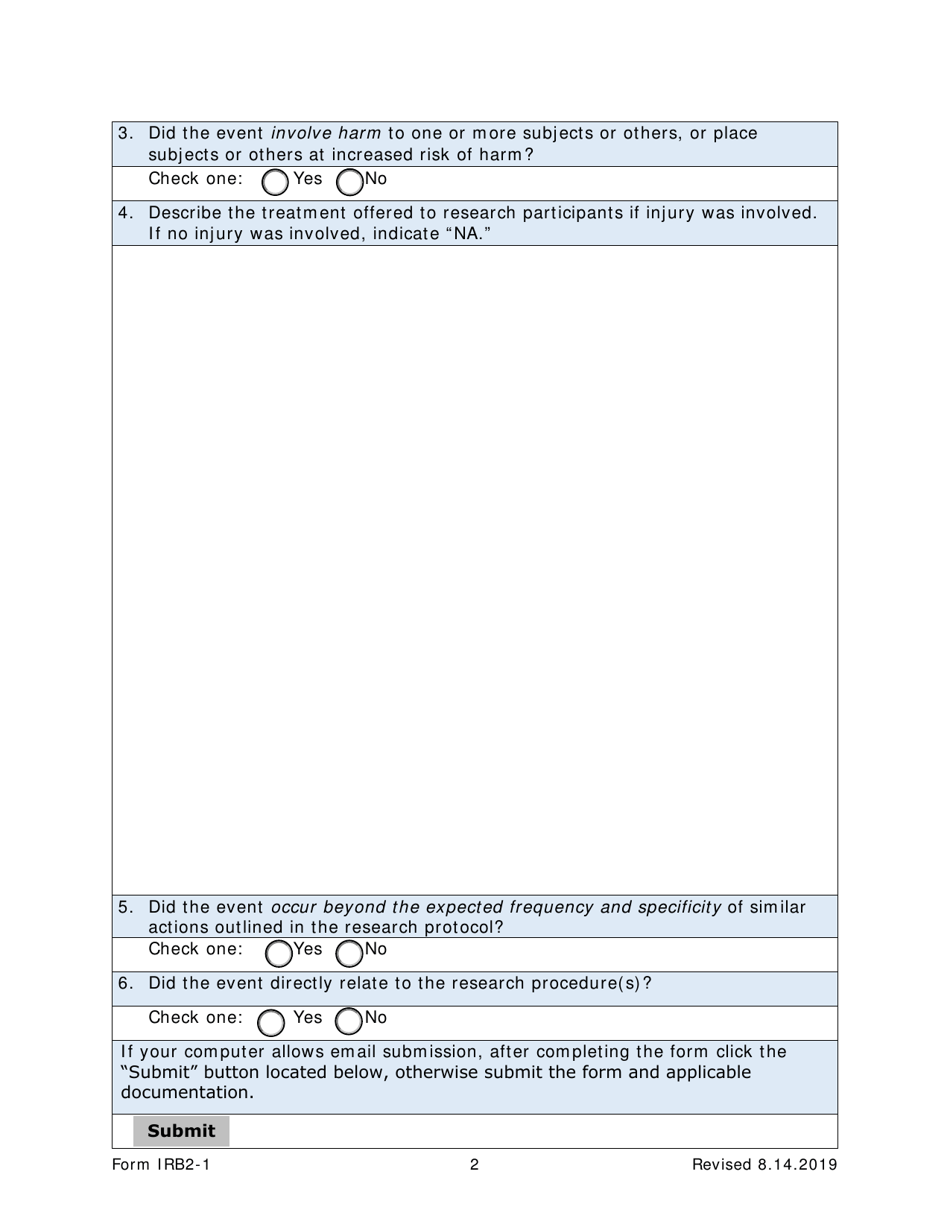
Form IRB2-1 Irb2 Adverse Event & Unanticipated Problem Report - Texas
What Is Form IRB2-1?
This is a legal form that was released by the Texas Health and Human Services - a government authority operating within Texas. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is IRB2-1?
A: IRB2-1 is a form for reporting adverse events and unanticipated problems in Texas.
Q: What is the purpose of form IRB2-1?
A: The purpose of form IRB2-1 is to report adverse events and unanticipated problems related to research studies.
Q: Who is required to complete form IRB2-1?
A: Researchers conducting research studies in Texas are required to complete form IRB2-1.
Q: What should be reported on form IRB2-1?
A: Form IRB2-1 should be used to report any adverse events or unanticipated problems that occur during the course of a research study.
Q: How should form IRB2-1 be submitted?
A: Form IRB2-1 should be submitted to the Institutional Review Board (IRB) of the institution where the research study is being conducted.
Form Details:
- Released on August 14, 2019;
- The latest edition provided by the Texas Health and Human Services;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form IRB2-1 by clicking the link below or browse more documents and templates provided by the Texas Health and Human Services.