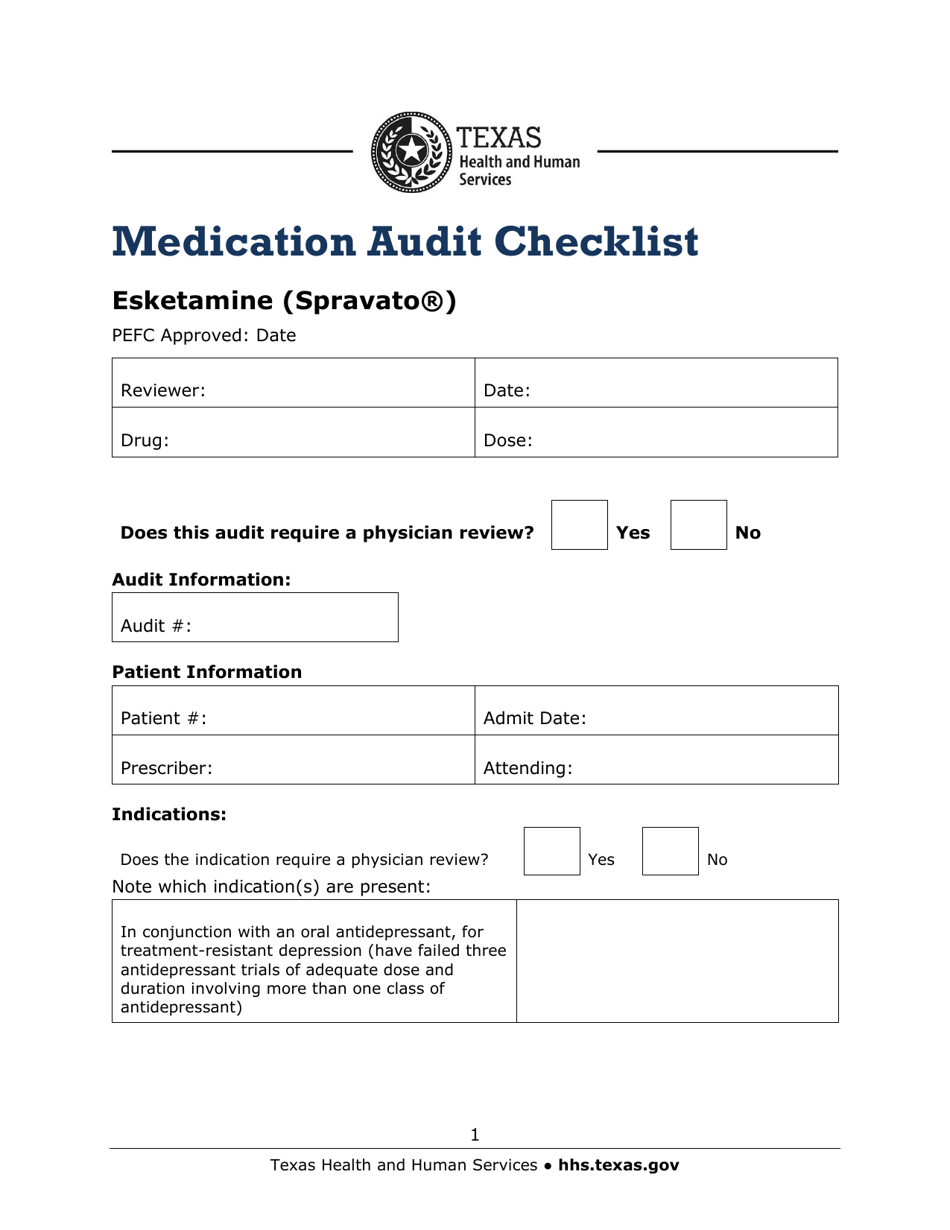
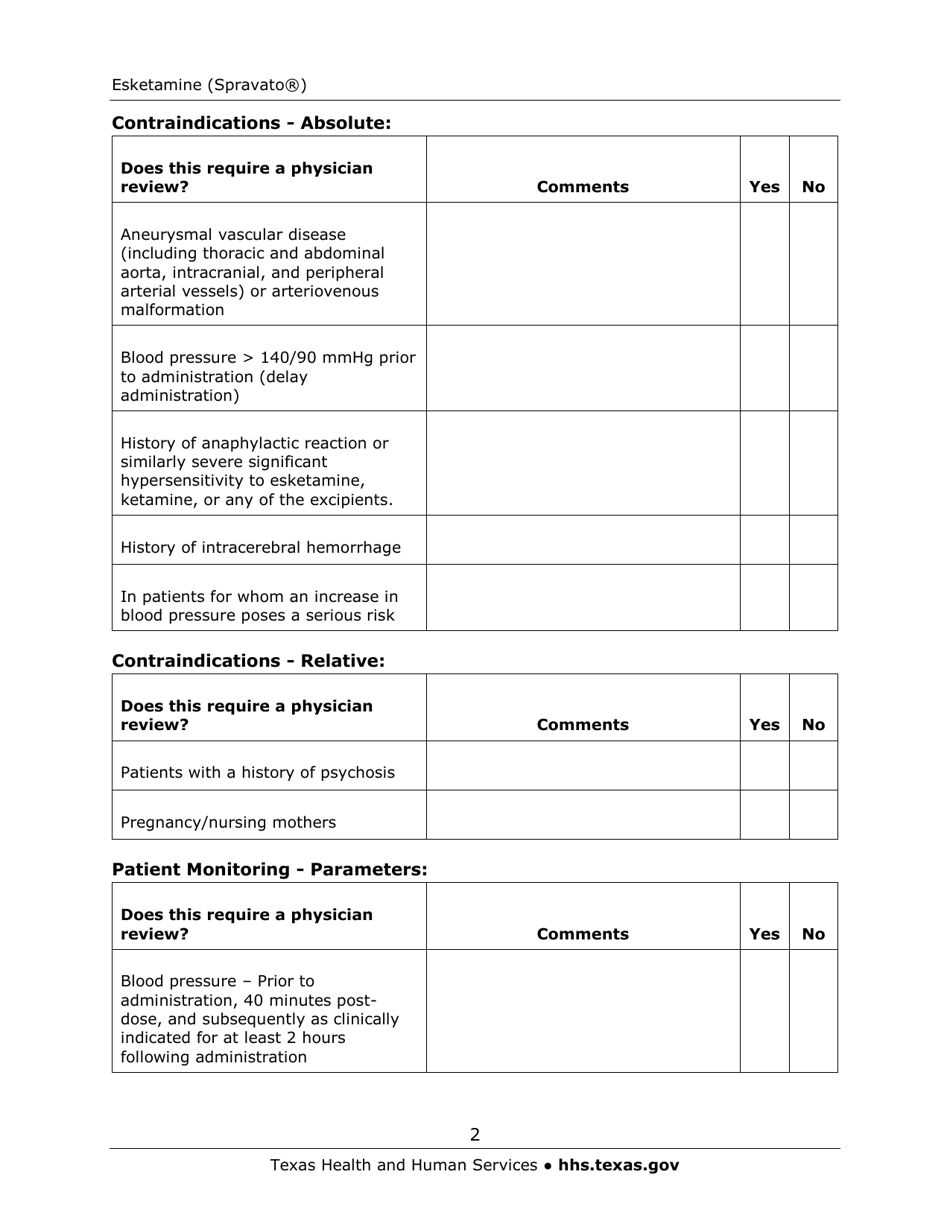
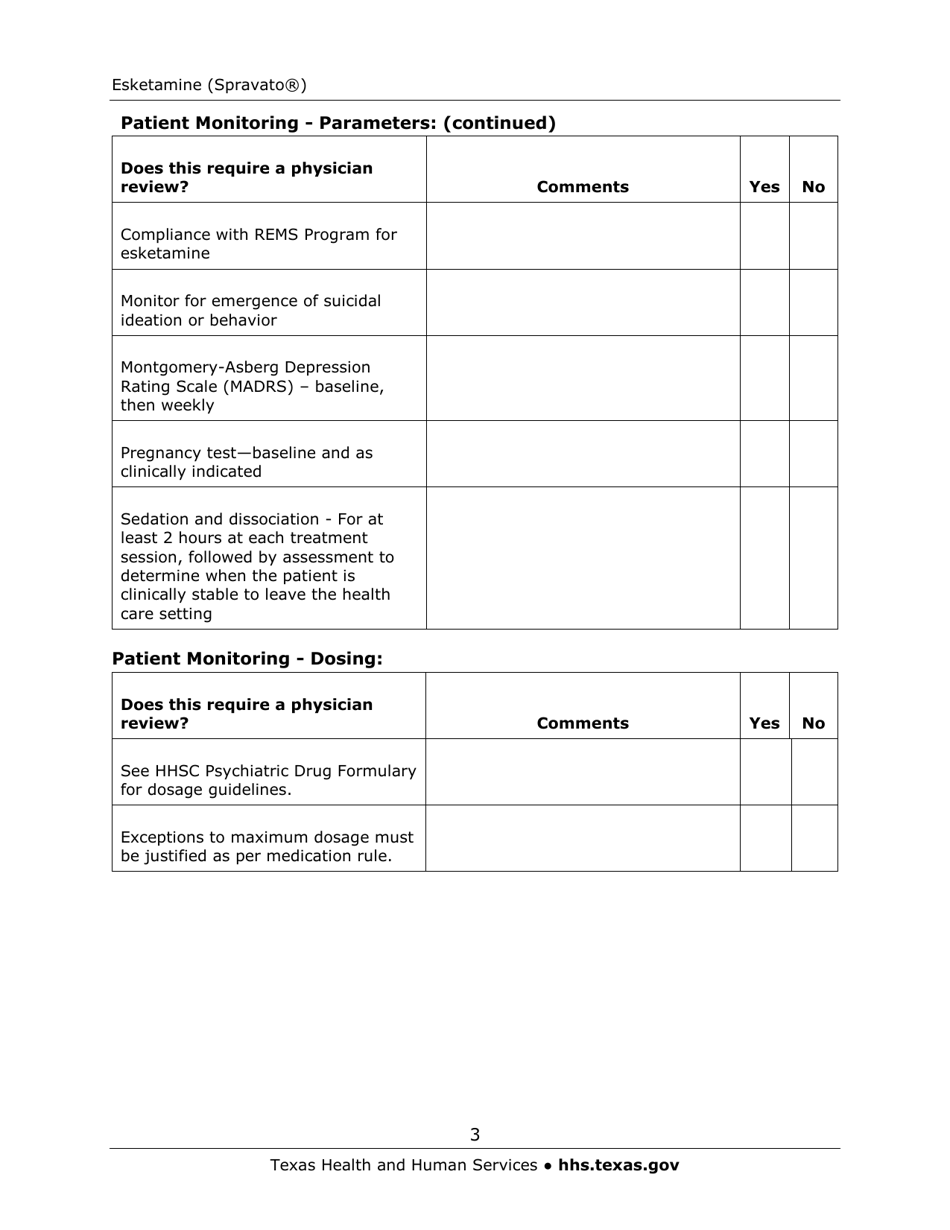
Medication Audit Checklist - Esketamine (Spravato) - Texas
Medication Audit Checklist - Esketamine (Spravato) is a legal document that was released by the Texas Health and Human Services - a government authority operating within Texas.
FAQ
Q: What is the medication Esketamine (Spravato)?
A: Esketamine, brand name Spravato, is a medication used to treat depression.
Q: Is Esketamine approved by the FDA?
A: Yes, Esketamine (Spravato) is approved by the FDA as a treatment for depression.
Q: How is Esketamine administered?
A: Esketamine is administered as a nasal spray.
Q: Who can prescribe Esketamine?
A: Only healthcare providers enrolled in the Spravato REMS (Risk Evaluation and Mitigation Strategy) program can prescribe Esketamine.
Q: Is Esketamine covered by insurance?
A: Coverage for Esketamine may vary depending on your insurance provider and specific plan. It is recommended to contact your insurance company for more information.
Q: What are the potential side effects of Esketamine?
A: Common side effects of Esketamine may include nausea, dizziness, dissociation, headache, and increased blood pressure.
Q: How often is Esketamine administered?
A: The initial recommended dosage is twice a week for the first month, followed by once weekly or every other week depending on the individual's response.
Q: Is Esketamine suitable for everyone with depression?
A: Esketamine is specifically indicated for adults with treatment-resistant depression who have not responded to other antidepressant medications.
Q: Can Esketamine be used in combination with other antidepressants?
A: Yes, Esketamine treatment may be used in combination with an oral antidepressant.
Form Details:
- The latest edition currently provided by the Texas Health and Human Services;
- Ready to use and print;
- Easy to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a printable version of the form by clicking the link below or browse more documents and templates provided by the Texas Health and Human Services.